TREATMENT AND OUTCOME PATTERNS IN PATIENTS WITH RELAPSED WALDENSTRÖM’S MACROGLOBULINEMIA: DEVELOPMENT OF A LARGE OBSERVATIONAL PAN-EUROPEAN DATA PLATFORM
(Abstract release date: 05/19/16)
EHA Library. Buske C. 06/09/16; 132824; E1275

Prof. Christian Buske
Contributions
Contributions
Abstract
Abstract: E1275
Type: Eposter Presentation
Background
There are few randomized trials and no well-established treatment standards for Waldenström’s Macroglobulinemia (WM). Data on treatment choices and their outcome in patients (pts) outside clinical trials are lacking.
Aims
The goal of this observational chart review was to generate real-world data on diagnosis, treatment patterns, and outcomes for WM over a decade in a large effort involving several European countries.
Methods
Physicians completed retrospective electronic records for pts who fit the following criteria: confirmed WM, symptomatic disease at treatment initiation, frontline treatment initiated between Jan 2000-Dec 2010, treatment with at least one salvage regimen (excluding maintenance therapy), and availability of clinical/biologic evaluation at diagnosis or initial therapy. Study endpoints included initial/subsequent lines of treatment, progression-free survival (PFS), and overall survival (OS). The number of pt records per country was prespecified to balance distribution between countries.
Results
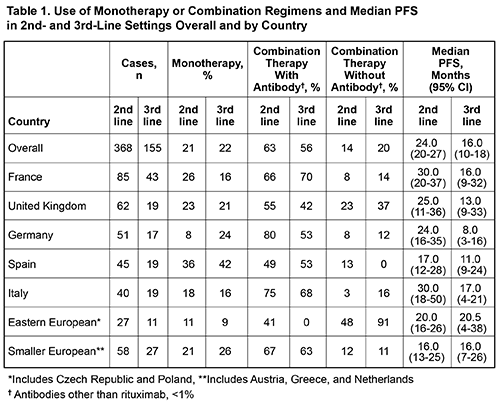
Electronic records were reviewed for 368 pts from France (n=85), United Kingdom (n=62), Germany (n=51), Spain (n=45), Italy (n=40), Greece (n=25), Netherlands (n=21), Poland (n=14), Czech Republic (n=13), and Austria (n=12). Data were summarized across 2nd/3rd-lines for 368/155 pts. Median age at initiation of frontline treatment was 63 yr (range, 29-89); 61% were male. Reasons for initiating treatment were cytopenias (77%; anemia [75%]), constitutional symptoms (56%), IgM-related symptoms (55%), and organomegaly (26%). Choice of therapy varied with line of treatment and age; monotherapy fell from 35% in frontline to 21%/22% in 2nd/3rd-lines (Table 1) and age ≥70 was associated with greater use of monotherapy (40%) vs combination therapy (29%) in the frontline. Combination therapy with rituximab increased from 36% in frontline to 63%/56% in 2nd/3rd-lines. Across all lines, rituximab and cyclophosphamide were the most common agents, excluding steroids, used as monotherapy or in any combination. Median PFS decreased with successive lines of treatment from 31 mo for frontline to 24/16 mo for 2nd/3rd-lines. Median PFS varied by country and choice of agents (Table 1), but was similar for pts <50 and ≥50 y. Improved median PFS was observed for pts who received rituximab in 2nd-line vs pts who did not (26 vs 19 mo, P=0.014). Treatment outcomes were similar in 3rd-line with regards to rituximab use (15 vs 16 mo, P=0.69). The use of rituximab in the frontline did not affect median PFS of subsequent lines of therapy (2nd-line: 24 vs 23 mo, P=0.87; 3rd-line: 13 vs 16 mo, P=0.12). Median OS for the overall population was not reached, but was significantly lower in pts ≥75 yr (70 mo; P <0.0001) or in pts with high-risk IPSSWM risk score (91 mo; P=0.0014). The current data does not indicate a difference in OS with frontline rituximab use. Other malignancies were reported in 14% after receipt of at least one line of treatment for WM.
Conclusion
For WM pts treated in Europe, the most common reasons for initiating therapy are anemia and constitutional symptoms. Rituximab was the most commonly used agent across all lines of treatment and use of rituximab was associated with improvement in median PFS in the 2nd-line. Outside clinical trials, monotherapy is widely used even at first relapse with notable differences between countries. This large observational dataset will be an important tool to promote understanding of treatment practices and survival of WM pts outside of a clinical trial setting.
Session topic: E-poster
Keyword(s): B cell lymphoma, Clinical outcome, Waldenstrom's macroglobulinemia
Type: Eposter Presentation
Background
There are few randomized trials and no well-established treatment standards for Waldenström’s Macroglobulinemia (WM). Data on treatment choices and their outcome in patients (pts) outside clinical trials are lacking.
Aims
The goal of this observational chart review was to generate real-world data on diagnosis, treatment patterns, and outcomes for WM over a decade in a large effort involving several European countries.
Methods
Physicians completed retrospective electronic records for pts who fit the following criteria: confirmed WM, symptomatic disease at treatment initiation, frontline treatment initiated between Jan 2000-Dec 2010, treatment with at least one salvage regimen (excluding maintenance therapy), and availability of clinical/biologic evaluation at diagnosis or initial therapy. Study endpoints included initial/subsequent lines of treatment, progression-free survival (PFS), and overall survival (OS). The number of pt records per country was prespecified to balance distribution between countries.
Results
Electronic records were reviewed for 368 pts from France (n=85), United Kingdom (n=62), Germany (n=51), Spain (n=45), Italy (n=40), Greece (n=25), Netherlands (n=21), Poland (n=14), Czech Republic (n=13), and Austria (n=12). Data were summarized across 2nd/3rd-lines for 368/155 pts. Median age at initiation of frontline treatment was 63 yr (range, 29-89); 61% were male. Reasons for initiating treatment were cytopenias (77%; anemia [75%]), constitutional symptoms (56%), IgM-related symptoms (55%), and organomegaly (26%). Choice of therapy varied with line of treatment and age; monotherapy fell from 35% in frontline to 21%/22% in 2nd/3rd-lines (Table 1) and age ≥70 was associated with greater use of monotherapy (40%) vs combination therapy (29%) in the frontline. Combination therapy with rituximab increased from 36% in frontline to 63%/56% in 2nd/3rd-lines. Across all lines, rituximab and cyclophosphamide were the most common agents, excluding steroids, used as monotherapy or in any combination. Median PFS decreased with successive lines of treatment from 31 mo for frontline to 24/16 mo for 2nd/3rd-lines. Median PFS varied by country and choice of agents (Table 1), but was similar for pts <50 and ≥50 y. Improved median PFS was observed for pts who received rituximab in 2nd-line vs pts who did not (26 vs 19 mo, P=0.014). Treatment outcomes were similar in 3rd-line with regards to rituximab use (15 vs 16 mo, P=0.69). The use of rituximab in the frontline did not affect median PFS of subsequent lines of therapy (2nd-line: 24 vs 23 mo, P=0.87; 3rd-line: 13 vs 16 mo, P=0.12). Median OS for the overall population was not reached, but was significantly lower in pts ≥75 yr (70 mo; P <0.0001) or in pts with high-risk IPSSWM risk score (91 mo; P=0.0014). The current data does not indicate a difference in OS with frontline rituximab use. Other malignancies were reported in 14% after receipt of at least one line of treatment for WM.
Conclusion
For WM pts treated in Europe, the most common reasons for initiating therapy are anemia and constitutional symptoms. Rituximab was the most commonly used agent across all lines of treatment and use of rituximab was associated with improvement in median PFS in the 2nd-line. Outside clinical trials, monotherapy is widely used even at first relapse with notable differences between countries. This large observational dataset will be an important tool to promote understanding of treatment practices and survival of WM pts outside of a clinical trial setting.

Session topic: E-poster
Keyword(s): B cell lymphoma, Clinical outcome, Waldenstrom's macroglobulinemia
Abstract: E1275
Type: Eposter Presentation
Background
There are few randomized trials and no well-established treatment standards for Waldenström’s Macroglobulinemia (WM). Data on treatment choices and their outcome in patients (pts) outside clinical trials are lacking.
Aims
The goal of this observational chart review was to generate real-world data on diagnosis, treatment patterns, and outcomes for WM over a decade in a large effort involving several European countries.
Methods
Physicians completed retrospective electronic records for pts who fit the following criteria: confirmed WM, symptomatic disease at treatment initiation, frontline treatment initiated between Jan 2000-Dec 2010, treatment with at least one salvage regimen (excluding maintenance therapy), and availability of clinical/biologic evaluation at diagnosis or initial therapy. Study endpoints included initial/subsequent lines of treatment, progression-free survival (PFS), and overall survival (OS). The number of pt records per country was prespecified to balance distribution between countries.
Results
Electronic records were reviewed for 368 pts from France (n=85), United Kingdom (n=62), Germany (n=51), Spain (n=45), Italy (n=40), Greece (n=25), Netherlands (n=21), Poland (n=14), Czech Republic (n=13), and Austria (n=12). Data were summarized across 2nd/3rd-lines for 368/155 pts. Median age at initiation of frontline treatment was 63 yr (range, 29-89); 61% were male. Reasons for initiating treatment were cytopenias (77%; anemia [75%]), constitutional symptoms (56%), IgM-related symptoms (55%), and organomegaly (26%). Choice of therapy varied with line of treatment and age; monotherapy fell from 35% in frontline to 21%/22% in 2nd/3rd-lines (Table 1) and age ≥70 was associated with greater use of monotherapy (40%) vs combination therapy (29%) in the frontline. Combination therapy with rituximab increased from 36% in frontline to 63%/56% in 2nd/3rd-lines. Across all lines, rituximab and cyclophosphamide were the most common agents, excluding steroids, used as monotherapy or in any combination. Median PFS decreased with successive lines of treatment from 31 mo for frontline to 24/16 mo for 2nd/3rd-lines. Median PFS varied by country and choice of agents (Table 1), but was similar for pts <50 and ≥50 y. Improved median PFS was observed for pts who received rituximab in 2nd-line vs pts who did not (26 vs 19 mo, P=0.014). Treatment outcomes were similar in 3rd-line with regards to rituximab use (15 vs 16 mo, P=0.69). The use of rituximab in the frontline did not affect median PFS of subsequent lines of therapy (2nd-line: 24 vs 23 mo, P=0.87; 3rd-line: 13 vs 16 mo, P=0.12). Median OS for the overall population was not reached, but was significantly lower in pts ≥75 yr (70 mo; P <0.0001) or in pts with high-risk IPSSWM risk score (91 mo; P=0.0014). The current data does not indicate a difference in OS with frontline rituximab use. Other malignancies were reported in 14% after receipt of at least one line of treatment for WM.
Conclusion
For WM pts treated in Europe, the most common reasons for initiating therapy are anemia and constitutional symptoms. Rituximab was the most commonly used agent across all lines of treatment and use of rituximab was associated with improvement in median PFS in the 2nd-line. Outside clinical trials, monotherapy is widely used even at first relapse with notable differences between countries. This large observational dataset will be an important tool to promote understanding of treatment practices and survival of WM pts outside of a clinical trial setting.

Session topic: E-poster
Keyword(s): B cell lymphoma, Clinical outcome, Waldenstrom's macroglobulinemia
Type: Eposter Presentation
Background
There are few randomized trials and no well-established treatment standards for Waldenström’s Macroglobulinemia (WM). Data on treatment choices and their outcome in patients (pts) outside clinical trials are lacking.
Aims
The goal of this observational chart review was to generate real-world data on diagnosis, treatment patterns, and outcomes for WM over a decade in a large effort involving several European countries.
Methods
Physicians completed retrospective electronic records for pts who fit the following criteria: confirmed WM, symptomatic disease at treatment initiation, frontline treatment initiated between Jan 2000-Dec 2010, treatment with at least one salvage regimen (excluding maintenance therapy), and availability of clinical/biologic evaluation at diagnosis or initial therapy. Study endpoints included initial/subsequent lines of treatment, progression-free survival (PFS), and overall survival (OS). The number of pt records per country was prespecified to balance distribution between countries.
Results
Electronic records were reviewed for 368 pts from France (n=85), United Kingdom (n=62), Germany (n=51), Spain (n=45), Italy (n=40), Greece (n=25), Netherlands (n=21), Poland (n=14), Czech Republic (n=13), and Austria (n=12). Data were summarized across 2nd/3rd-lines for 368/155 pts. Median age at initiation of frontline treatment was 63 yr (range, 29-89); 61% were male. Reasons for initiating treatment were cytopenias (77%; anemia [75%]), constitutional symptoms (56%), IgM-related symptoms (55%), and organomegaly (26%). Choice of therapy varied with line of treatment and age; monotherapy fell from 35% in frontline to 21%/22% in 2nd/3rd-lines (Table 1) and age ≥70 was associated with greater use of monotherapy (40%) vs combination therapy (29%) in the frontline. Combination therapy with rituximab increased from 36% in frontline to 63%/56% in 2nd/3rd-lines. Across all lines, rituximab and cyclophosphamide were the most common agents, excluding steroids, used as monotherapy or in any combination. Median PFS decreased with successive lines of treatment from 31 mo for frontline to 24/16 mo for 2nd/3rd-lines. Median PFS varied by country and choice of agents (Table 1), but was similar for pts <50 and ≥50 y. Improved median PFS was observed for pts who received rituximab in 2nd-line vs pts who did not (26 vs 19 mo, P=0.014). Treatment outcomes were similar in 3rd-line with regards to rituximab use (15 vs 16 mo, P=0.69). The use of rituximab in the frontline did not affect median PFS of subsequent lines of therapy (2nd-line: 24 vs 23 mo, P=0.87; 3rd-line: 13 vs 16 mo, P=0.12). Median OS for the overall population was not reached, but was significantly lower in pts ≥75 yr (70 mo; P <0.0001) or in pts with high-risk IPSSWM risk score (91 mo; P=0.0014). The current data does not indicate a difference in OS with frontline rituximab use. Other malignancies were reported in 14% after receipt of at least one line of treatment for WM.
Conclusion
For WM pts treated in Europe, the most common reasons for initiating therapy are anemia and constitutional symptoms. Rituximab was the most commonly used agent across all lines of treatment and use of rituximab was associated with improvement in median PFS in the 2nd-line. Outside clinical trials, monotherapy is widely used even at first relapse with notable differences between countries. This large observational dataset will be an important tool to promote understanding of treatment practices and survival of WM pts outside of a clinical trial setting.

Session topic: E-poster
Keyword(s): B cell lymphoma, Clinical outcome, Waldenstrom's macroglobulinemia
{{ help_message }}
{{filter}}


