CARFILZOMIB AND DEXAMETHASONE VS BORTEZOMIB AND DEXAMETHASONE IN PATIENTS WITH RELAPSED MULTIPLE MYELOMA: ANALYSIS OF THE PHASE 3 ENDEAVOR STUDY BY AGE SUBGROUP
(Abstract release date: 05/19/16)
EHA Library. Palumbo A. 06/09/16; 132823; E1274

Prof. Antonio Palumbo
Contributions
Contributions
Abstract
Abstract: E1274
Type: Eposter Presentation
Background
The selective proteasome inhibitor carfilzomib is approved in the United States (US) as a single agent for the treatment of relapsed and refractory multiple myeloma (MM) and in combination with dexamethasone for relapsed MM. It is also approved in the US and Europe in combination with lenalidomide and dexamethasone for the treatment of patients with relapsed MM (RMM). In the phase 3 study (ENDEAVOR; NCT01568866) carfilzomib and dexamethasone (Cd) doubled progression-free survival (PFS) compared with bortezomib and dexamethasone (Bd) in patients with RMM (median 18.7 vs 9.4 months; hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.44, 0.65; 1-sided p<0.0001).
Aims
A pre-planned subgroup analysis of the ENDEAVOR study according to patients’ age (<65, 65–74, and ≥75 years of age).
Methods
Adults with RMM (1–3 prior regimens) were randomized to either the Cd arm (carfilzomib 30-min intravenous [IV] infusion on days [D] 1, 2, 8, 9, 15, and 16 [20mg/m2 on D1, 2 in cycle 1; 56mg/m2 thereafter]) and dexamethasone (20mg on D1, 2, 8, 9, 15, 16, 22 and 23 of 28-day cycles) or the Bd arm (bortezomib 1.3mg/m2 IV or subcutaneous on D1, 4, 8, and 11) and dexamethasone (20mg on D1, 2, 4, 5, 8, 9, 11, and 12 of 21-day cycles). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS and secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), safety, and rate of peripheral neuropathy (PN).
Results
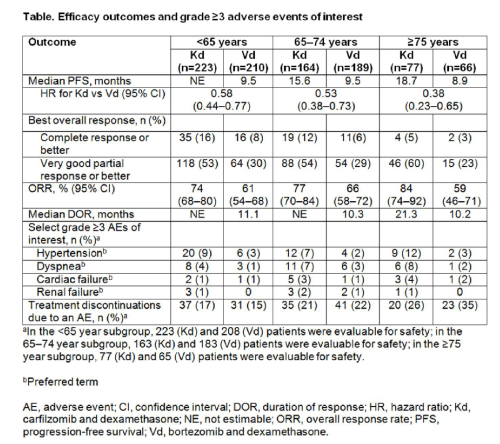
Of the 929 patients enrolled, in the <65 years subgroup: 223 patients received Cd and 210 received Bd; in the 65–74 years subgroup: Cd, n=164; Bd, n=189; and the ≥75 years subgroup: Cd, n=77; Bd, n=66. Patient and disease characteristics were balanced across treatment arms within each age subgroup. For all age subgroups, median PFS was improved with Cd vs Bd (<65 years: not estimable vs 9.5 months [HR, 0.58; 95% CI, 0.44, 0.77]; 65–74 years: 15.6 months vs 9.5 months [HR, 0.53; 95% CI, 0.38, 0.73]; ≥75 years: 18.7 months vs 8.9 months [HR, 0.38; 95% CI, 0.23, 0.65]) (Table). ORRs in each age group were also consistently higher in the Cd arm versus the Bd arm (<65 years: 74% vs 61% [odds ratio, 1.82; 95% CI, 1.21, 2.74]; 65–74 years: 77% vs 66% [odds ratio, 1.80; 95% CI, 1.12, 2.89]; ≥75 years: 84% vs 59% [odds ratio, 3.75; 95% CI, 1.71, 8.24]). Grade ≥3 hypertension, dyspnea, cardiac failure, renal failure were more common with Cd vs Bd. However, rates of grade ≥2 PN were lower in the Cd arm compared with the Bd arm (<65 years: 6% vs 27% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; 65–74 years: 8% vs 34% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; ≥75 years: 3% vs 43% [odds ratio, 0.04; 95% CI, 0.01, 0.16]). Deaths within 30 days post-study drug due to adverse events occurred at a similar frequency in the Cd and Bd arms (<65 years: 3% vs 3%; 65–74 years: 5% vs 3%; ≥75 years: 4% vs 5%).
Conclusion
Cd significantly improved PFS and ORR compared with Bd across all age subgroups. A trend toward a greater improvement was observed in the eldest-age subgroup (≥75 years) versus the two younger-age subgroups (<65 and 65–74 years). In the eldest-age subgroup treatment with Cd was associated with an increased incidence of select grade ≥3 adverse events of interest, including cardiac failure and hypertension, compared with treatment with Cd in the younger-age subgroups. Hypertension is a common and manageable complication in elderly patients and should be monitored. Cd has a favorable benefit-risk profile in patients with RMM, irrespective of age.
Session topic: E-poster
Keyword(s): Elderly, Multiple myeloma, Proteasome inhibitor, Relapse
Type: Eposter Presentation
Background
The selective proteasome inhibitor carfilzomib is approved in the United States (US) as a single agent for the treatment of relapsed and refractory multiple myeloma (MM) and in combination with dexamethasone for relapsed MM. It is also approved in the US and Europe in combination with lenalidomide and dexamethasone for the treatment of patients with relapsed MM (RMM). In the phase 3 study (ENDEAVOR; NCT01568866) carfilzomib and dexamethasone (Cd) doubled progression-free survival (PFS) compared with bortezomib and dexamethasone (Bd) in patients with RMM (median 18.7 vs 9.4 months; hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.44, 0.65; 1-sided p<0.0001).
Aims
A pre-planned subgroup analysis of the ENDEAVOR study according to patients’ age (<65, 65–74, and ≥75 years of age).
Methods
Adults with RMM (1–3 prior regimens) were randomized to either the Cd arm (carfilzomib 30-min intravenous [IV] infusion on days [D] 1, 2, 8, 9, 15, and 16 [20mg/m2 on D1, 2 in cycle 1; 56mg/m2 thereafter]) and dexamethasone (20mg on D1, 2, 8, 9, 15, 16, 22 and 23 of 28-day cycles) or the Bd arm (bortezomib 1.3mg/m2 IV or subcutaneous on D1, 4, 8, and 11) and dexamethasone (20mg on D1, 2, 4, 5, 8, 9, 11, and 12 of 21-day cycles). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS and secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), safety, and rate of peripheral neuropathy (PN).
Results
Of the 929 patients enrolled, in the <65 years subgroup: 223 patients received Cd and 210 received Bd; in the 65–74 years subgroup: Cd, n=164; Bd, n=189; and the ≥75 years subgroup: Cd, n=77; Bd, n=66. Patient and disease characteristics were balanced across treatment arms within each age subgroup. For all age subgroups, median PFS was improved with Cd vs Bd (<65 years: not estimable vs 9.5 months [HR, 0.58; 95% CI, 0.44, 0.77]; 65–74 years: 15.6 months vs 9.5 months [HR, 0.53; 95% CI, 0.38, 0.73]; ≥75 years: 18.7 months vs 8.9 months [HR, 0.38; 95% CI, 0.23, 0.65]) (Table). ORRs in each age group were also consistently higher in the Cd arm versus the Bd arm (<65 years: 74% vs 61% [odds ratio, 1.82; 95% CI, 1.21, 2.74]; 65–74 years: 77% vs 66% [odds ratio, 1.80; 95% CI, 1.12, 2.89]; ≥75 years: 84% vs 59% [odds ratio, 3.75; 95% CI, 1.71, 8.24]). Grade ≥3 hypertension, dyspnea, cardiac failure, renal failure were more common with Cd vs Bd. However, rates of grade ≥2 PN were lower in the Cd arm compared with the Bd arm (<65 years: 6% vs 27% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; 65–74 years: 8% vs 34% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; ≥75 years: 3% vs 43% [odds ratio, 0.04; 95% CI, 0.01, 0.16]). Deaths within 30 days post-study drug due to adverse events occurred at a similar frequency in the Cd and Bd arms (<65 years: 3% vs 3%; 65–74 years: 5% vs 3%; ≥75 years: 4% vs 5%).
Conclusion
Cd significantly improved PFS and ORR compared with Bd across all age subgroups. A trend toward a greater improvement was observed in the eldest-age subgroup (≥75 years) versus the two younger-age subgroups (<65 and 65–74 years). In the eldest-age subgroup treatment with Cd was associated with an increased incidence of select grade ≥3 adverse events of interest, including cardiac failure and hypertension, compared with treatment with Cd in the younger-age subgroups. Hypertension is a common and manageable complication in elderly patients and should be monitored. Cd has a favorable benefit-risk profile in patients with RMM, irrespective of age.

Session topic: E-poster
Keyword(s): Elderly, Multiple myeloma, Proteasome inhibitor, Relapse
Abstract: E1274
Type: Eposter Presentation
Background
The selective proteasome inhibitor carfilzomib is approved in the United States (US) as a single agent for the treatment of relapsed and refractory multiple myeloma (MM) and in combination with dexamethasone for relapsed MM. It is also approved in the US and Europe in combination with lenalidomide and dexamethasone for the treatment of patients with relapsed MM (RMM). In the phase 3 study (ENDEAVOR; NCT01568866) carfilzomib and dexamethasone (Cd) doubled progression-free survival (PFS) compared with bortezomib and dexamethasone (Bd) in patients with RMM (median 18.7 vs 9.4 months; hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.44, 0.65; 1-sided p<0.0001).
Aims
A pre-planned subgroup analysis of the ENDEAVOR study according to patients’ age (<65, 65–74, and ≥75 years of age).
Methods
Adults with RMM (1–3 prior regimens) were randomized to either the Cd arm (carfilzomib 30-min intravenous [IV] infusion on days [D] 1, 2, 8, 9, 15, and 16 [20mg/m2 on D1, 2 in cycle 1; 56mg/m2 thereafter]) and dexamethasone (20mg on D1, 2, 8, 9, 15, 16, 22 and 23 of 28-day cycles) or the Bd arm (bortezomib 1.3mg/m2 IV or subcutaneous on D1, 4, 8, and 11) and dexamethasone (20mg on D1, 2, 4, 5, 8, 9, 11, and 12 of 21-day cycles). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS and secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), safety, and rate of peripheral neuropathy (PN).
Results
Of the 929 patients enrolled, in the <65 years subgroup: 223 patients received Cd and 210 received Bd; in the 65–74 years subgroup: Cd, n=164; Bd, n=189; and the ≥75 years subgroup: Cd, n=77; Bd, n=66. Patient and disease characteristics were balanced across treatment arms within each age subgroup. For all age subgroups, median PFS was improved with Cd vs Bd (<65 years: not estimable vs 9.5 months [HR, 0.58; 95% CI, 0.44, 0.77]; 65–74 years: 15.6 months vs 9.5 months [HR, 0.53; 95% CI, 0.38, 0.73]; ≥75 years: 18.7 months vs 8.9 months [HR, 0.38; 95% CI, 0.23, 0.65]) (Table). ORRs in each age group were also consistently higher in the Cd arm versus the Bd arm (<65 years: 74% vs 61% [odds ratio, 1.82; 95% CI, 1.21, 2.74]; 65–74 years: 77% vs 66% [odds ratio, 1.80; 95% CI, 1.12, 2.89]; ≥75 years: 84% vs 59% [odds ratio, 3.75; 95% CI, 1.71, 8.24]). Grade ≥3 hypertension, dyspnea, cardiac failure, renal failure were more common with Cd vs Bd. However, rates of grade ≥2 PN were lower in the Cd arm compared with the Bd arm (<65 years: 6% vs 27% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; 65–74 years: 8% vs 34% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; ≥75 years: 3% vs 43% [odds ratio, 0.04; 95% CI, 0.01, 0.16]). Deaths within 30 days post-study drug due to adverse events occurred at a similar frequency in the Cd and Bd arms (<65 years: 3% vs 3%; 65–74 years: 5% vs 3%; ≥75 years: 4% vs 5%).
Conclusion
Cd significantly improved PFS and ORR compared with Bd across all age subgroups. A trend toward a greater improvement was observed in the eldest-age subgroup (≥75 years) versus the two younger-age subgroups (<65 and 65–74 years). In the eldest-age subgroup treatment with Cd was associated with an increased incidence of select grade ≥3 adverse events of interest, including cardiac failure and hypertension, compared with treatment with Cd in the younger-age subgroups. Hypertension is a common and manageable complication in elderly patients and should be monitored. Cd has a favorable benefit-risk profile in patients with RMM, irrespective of age.

Session topic: E-poster
Keyword(s): Elderly, Multiple myeloma, Proteasome inhibitor, Relapse
Type: Eposter Presentation
Background
The selective proteasome inhibitor carfilzomib is approved in the United States (US) as a single agent for the treatment of relapsed and refractory multiple myeloma (MM) and in combination with dexamethasone for relapsed MM. It is also approved in the US and Europe in combination with lenalidomide and dexamethasone for the treatment of patients with relapsed MM (RMM). In the phase 3 study (ENDEAVOR; NCT01568866) carfilzomib and dexamethasone (Cd) doubled progression-free survival (PFS) compared with bortezomib and dexamethasone (Bd) in patients with RMM (median 18.7 vs 9.4 months; hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.44, 0.65; 1-sided p<0.0001).
Aims
A pre-planned subgroup analysis of the ENDEAVOR study according to patients’ age (<65, 65–74, and ≥75 years of age).
Methods
Adults with RMM (1–3 prior regimens) were randomized to either the Cd arm (carfilzomib 30-min intravenous [IV] infusion on days [D] 1, 2, 8, 9, 15, and 16 [20mg/m2 on D1, 2 in cycle 1; 56mg/m2 thereafter]) and dexamethasone (20mg on D1, 2, 8, 9, 15, 16, 22 and 23 of 28-day cycles) or the Bd arm (bortezomib 1.3mg/m2 IV or subcutaneous on D1, 4, 8, and 11) and dexamethasone (20mg on D1, 2, 4, 5, 8, 9, 11, and 12 of 21-day cycles). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS and secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), safety, and rate of peripheral neuropathy (PN).
Results
Of the 929 patients enrolled, in the <65 years subgroup: 223 patients received Cd and 210 received Bd; in the 65–74 years subgroup: Cd, n=164; Bd, n=189; and the ≥75 years subgroup: Cd, n=77; Bd, n=66. Patient and disease characteristics were balanced across treatment arms within each age subgroup. For all age subgroups, median PFS was improved with Cd vs Bd (<65 years: not estimable vs 9.5 months [HR, 0.58; 95% CI, 0.44, 0.77]; 65–74 years: 15.6 months vs 9.5 months [HR, 0.53; 95% CI, 0.38, 0.73]; ≥75 years: 18.7 months vs 8.9 months [HR, 0.38; 95% CI, 0.23, 0.65]) (Table). ORRs in each age group were also consistently higher in the Cd arm versus the Bd arm (<65 years: 74% vs 61% [odds ratio, 1.82; 95% CI, 1.21, 2.74]; 65–74 years: 77% vs 66% [odds ratio, 1.80; 95% CI, 1.12, 2.89]; ≥75 years: 84% vs 59% [odds ratio, 3.75; 95% CI, 1.71, 8.24]). Grade ≥3 hypertension, dyspnea, cardiac failure, renal failure were more common with Cd vs Bd. However, rates of grade ≥2 PN were lower in the Cd arm compared with the Bd arm (<65 years: 6% vs 27% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; 65–74 years: 8% vs 34% [odds ratio, 0.17; 95% CI, 0.09, 0.32]; ≥75 years: 3% vs 43% [odds ratio, 0.04; 95% CI, 0.01, 0.16]). Deaths within 30 days post-study drug due to adverse events occurred at a similar frequency in the Cd and Bd arms (<65 years: 3% vs 3%; 65–74 years: 5% vs 3%; ≥75 years: 4% vs 5%).
Conclusion
Cd significantly improved PFS and ORR compared with Bd across all age subgroups. A trend toward a greater improvement was observed in the eldest-age subgroup (≥75 years) versus the two younger-age subgroups (<65 and 65–74 years). In the eldest-age subgroup treatment with Cd was associated with an increased incidence of select grade ≥3 adverse events of interest, including cardiac failure and hypertension, compared with treatment with Cd in the younger-age subgroups. Hypertension is a common and manageable complication in elderly patients and should be monitored. Cd has a favorable benefit-risk profile in patients with RMM, irrespective of age.

Session topic: E-poster
Keyword(s): Elderly, Multiple myeloma, Proteasome inhibitor, Relapse
{{ help_message }}
{{filter}}


