CARFILZOMIB AND DEXAMETHASONE VS BORTEZOMIB AND DEXAMETHASONE: SUBGROUP ANALYSIS OF PATIENTS WITH RELAPSED MULTIPLE MYELOMA BY BASELINE CYTOGENETIC RISK STATUS (PHASE 3 ENDEAVOR STUDY)
(Abstract release date: 05/19/16)
EHA Library. Goldschmidt H. 06/09/16; 132816; E1267

Prof. Dr. Hartmut Goldschmidt
Contributions
Contributions
Abstract
Abstract: E1267
Type: Eposter Presentation
Background
In a previous study, single-agent carfilzomib demonstrated activity in patients with relapsed and refractory multiple myeloma with high-risk cytogenetic abnormalities. In the phase 3 study (NCT01568866; N=929 patients) carfilzomib plus dexamethasone (Cd) significantly improved progression-free survival (PFS) by 2-fold compared with bortezomib/dexamethasone (Bd) in patients with relapsed multiple myeloma (RMM) (18.7 months versus 9.4 months; hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.44–0.65; P<.0001).
Aims
A pre-planned subgroup analysis of efficacy and safety outcomes in patients treated with Cd vs Bd according to patients’ baseline cytogenetic risk status.
Methods
Adults with RMM who had received 1–3 prior lines of therapy received either carfilzomib (30-minute intravenous [IV] infusion on days 1, 2, 8, 9, 15, and 16 [20mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter]) and dexamethasone (20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle) (Cd) or bortezomib (1.3mg/m2 IV bolus or subcutaneous injection on days 1, 4, 8, and 11) and dexamethasone (20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle) (Bd). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety. The high-risk group was defined (using fluorescence in situ hybridization analysis of baseline bone marrow samples) as those patients with the genetic subtypes t(4;14) or t(14;16) in ≥10% of screened plasma cells or deletion 17p in ≥20% of screened plasma cells; the standard-risk group consisted of patients without these genetic subtypes.
Results
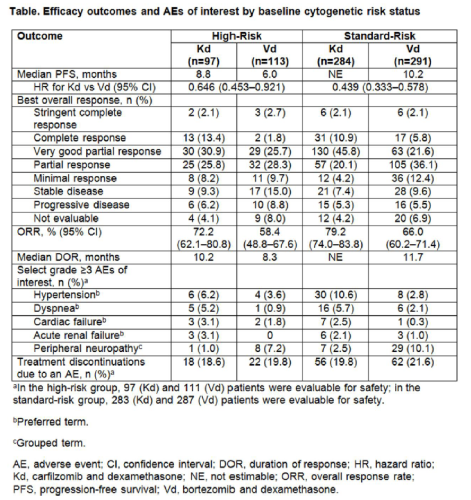
Of the 929 patients, 464 were randomized to receive Cd and 465 to receive Bd. Baseline cytogenetic risk status was balanced between the treatment arms (high-risk: Cd, 20.9%; Bd, 24.3%; standard-risk: Cd, 61.2%; Bd, 62.6%; unknown: Cd, 17.9%; Bd, 13.1%). Efficacy and safety end points by baseline cytogenetic risk status are presented in the Table. In the high-risk group, median PFS was 8.8 months (95% CI: 6.9, 11.3) for Cd vs 6.0 months (95% CI: 4.9, 8.1) for Bd (HR: 0.646; 95% CI: 0.453, 0.921). In the standard-risk group, median PFS was not estimable (NE) for Cd (95% CI: 18.7, NE) vs 10.2 months (95% CI: 9.3, 12.2) for Bd (HR: 0.439; 95% CI: 0.333, 0.578). ORRs (≥partial response) in the high-risk group were 72.2% (Cd) vs 58.4% (Bd) and 79.2% (Cd) vs 66.0% (Bd) in the standard-risk group. Median DOR in the high-risk group was 10.2 months for Cd vs 8.3 months for Bd. Median DOR in the standard-risk group was NE for Cd vs 11.7 months for Bd. Grade ≥3 adverse events (AEs) were reported at higher rates with Cd vs Bd in both the high- and standard-risk groups (70.1% vs 63.1% and 73.9% vs 68.3%). Grade ≥2 PN occurred less frequently with Cd vs Bd regardless of cytogenetic risk status (high-risk group: 3.1% vs 35.1%; odds ratio: 0.059; 95% CI: 0.018, 0.198 and standard-risk group: 6.4% vs 33.4%; odds ratio: 0.135; 95% CI: 0.079, 0.231).
Conclusion
Patients treated with Cd had a clinically meaningful improvement in PFS compared with Bd regardless of baseline cytogenetic risk status. Higher response rates, greater depth of response and longer DOR were also reported with Cd vs Bd. Cd had a favorable benefit–risk profile in patients with high-risk relapsed MM.
Session topic: E-poster
Keyword(s): Cytogenetics, Multiple myeloma, Proteasome inhibitor, Relapse
Type: Eposter Presentation
Background
In a previous study, single-agent carfilzomib demonstrated activity in patients with relapsed and refractory multiple myeloma with high-risk cytogenetic abnormalities. In the phase 3 study (NCT01568866; N=929 patients) carfilzomib plus dexamethasone (Cd) significantly improved progression-free survival (PFS) by 2-fold compared with bortezomib/dexamethasone (Bd) in patients with relapsed multiple myeloma (RMM) (18.7 months versus 9.4 months; hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.44–0.65; P<.0001).
Aims
A pre-planned subgroup analysis of efficacy and safety outcomes in patients treated with Cd vs Bd according to patients’ baseline cytogenetic risk status.
Methods
Adults with RMM who had received 1–3 prior lines of therapy received either carfilzomib (30-minute intravenous [IV] infusion on days 1, 2, 8, 9, 15, and 16 [20mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter]) and dexamethasone (20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle) (Cd) or bortezomib (1.3mg/m2 IV bolus or subcutaneous injection on days 1, 4, 8, and 11) and dexamethasone (20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle) (Bd). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety. The high-risk group was defined (using fluorescence in situ hybridization analysis of baseline bone marrow samples) as those patients with the genetic subtypes t(4;14) or t(14;16) in ≥10% of screened plasma cells or deletion 17p in ≥20% of screened plasma cells; the standard-risk group consisted of patients without these genetic subtypes.
Results
Of the 929 patients, 464 were randomized to receive Cd and 465 to receive Bd. Baseline cytogenetic risk status was balanced between the treatment arms (high-risk: Cd, 20.9%; Bd, 24.3%; standard-risk: Cd, 61.2%; Bd, 62.6%; unknown: Cd, 17.9%; Bd, 13.1%). Efficacy and safety end points by baseline cytogenetic risk status are presented in the Table. In the high-risk group, median PFS was 8.8 months (95% CI: 6.9, 11.3) for Cd vs 6.0 months (95% CI: 4.9, 8.1) for Bd (HR: 0.646; 95% CI: 0.453, 0.921). In the standard-risk group, median PFS was not estimable (NE) for Cd (95% CI: 18.7, NE) vs 10.2 months (95% CI: 9.3, 12.2) for Bd (HR: 0.439; 95% CI: 0.333, 0.578). ORRs (≥partial response) in the high-risk group were 72.2% (Cd) vs 58.4% (Bd) and 79.2% (Cd) vs 66.0% (Bd) in the standard-risk group. Median DOR in the high-risk group was 10.2 months for Cd vs 8.3 months for Bd. Median DOR in the standard-risk group was NE for Cd vs 11.7 months for Bd. Grade ≥3 adverse events (AEs) were reported at higher rates with Cd vs Bd in both the high- and standard-risk groups (70.1% vs 63.1% and 73.9% vs 68.3%). Grade ≥2 PN occurred less frequently with Cd vs Bd regardless of cytogenetic risk status (high-risk group: 3.1% vs 35.1%; odds ratio: 0.059; 95% CI: 0.018, 0.198 and standard-risk group: 6.4% vs 33.4%; odds ratio: 0.135; 95% CI: 0.079, 0.231).
Conclusion
Patients treated with Cd had a clinically meaningful improvement in PFS compared with Bd regardless of baseline cytogenetic risk status. Higher response rates, greater depth of response and longer DOR were also reported with Cd vs Bd. Cd had a favorable benefit–risk profile in patients with high-risk relapsed MM.

Session topic: E-poster
Keyword(s): Cytogenetics, Multiple myeloma, Proteasome inhibitor, Relapse
Abstract: E1267
Type: Eposter Presentation
Background
In a previous study, single-agent carfilzomib demonstrated activity in patients with relapsed and refractory multiple myeloma with high-risk cytogenetic abnormalities. In the phase 3 study (NCT01568866; N=929 patients) carfilzomib plus dexamethasone (Cd) significantly improved progression-free survival (PFS) by 2-fold compared with bortezomib/dexamethasone (Bd) in patients with relapsed multiple myeloma (RMM) (18.7 months versus 9.4 months; hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.44–0.65; P<.0001).
Aims
A pre-planned subgroup analysis of efficacy and safety outcomes in patients treated with Cd vs Bd according to patients’ baseline cytogenetic risk status.
Methods
Adults with RMM who had received 1–3 prior lines of therapy received either carfilzomib (30-minute intravenous [IV] infusion on days 1, 2, 8, 9, 15, and 16 [20mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter]) and dexamethasone (20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle) (Cd) or bortezomib (1.3mg/m2 IV bolus or subcutaneous injection on days 1, 4, 8, and 11) and dexamethasone (20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle) (Bd). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety. The high-risk group was defined (using fluorescence in situ hybridization analysis of baseline bone marrow samples) as those patients with the genetic subtypes t(4;14) or t(14;16) in ≥10% of screened plasma cells or deletion 17p in ≥20% of screened plasma cells; the standard-risk group consisted of patients without these genetic subtypes.
Results
Of the 929 patients, 464 were randomized to receive Cd and 465 to receive Bd. Baseline cytogenetic risk status was balanced between the treatment arms (high-risk: Cd, 20.9%; Bd, 24.3%; standard-risk: Cd, 61.2%; Bd, 62.6%; unknown: Cd, 17.9%; Bd, 13.1%). Efficacy and safety end points by baseline cytogenetic risk status are presented in the Table. In the high-risk group, median PFS was 8.8 months (95% CI: 6.9, 11.3) for Cd vs 6.0 months (95% CI: 4.9, 8.1) for Bd (HR: 0.646; 95% CI: 0.453, 0.921). In the standard-risk group, median PFS was not estimable (NE) for Cd (95% CI: 18.7, NE) vs 10.2 months (95% CI: 9.3, 12.2) for Bd (HR: 0.439; 95% CI: 0.333, 0.578). ORRs (≥partial response) in the high-risk group were 72.2% (Cd) vs 58.4% (Bd) and 79.2% (Cd) vs 66.0% (Bd) in the standard-risk group. Median DOR in the high-risk group was 10.2 months for Cd vs 8.3 months for Bd. Median DOR in the standard-risk group was NE for Cd vs 11.7 months for Bd. Grade ≥3 adverse events (AEs) were reported at higher rates with Cd vs Bd in both the high- and standard-risk groups (70.1% vs 63.1% and 73.9% vs 68.3%). Grade ≥2 PN occurred less frequently with Cd vs Bd regardless of cytogenetic risk status (high-risk group: 3.1% vs 35.1%; odds ratio: 0.059; 95% CI: 0.018, 0.198 and standard-risk group: 6.4% vs 33.4%; odds ratio: 0.135; 95% CI: 0.079, 0.231).
Conclusion
Patients treated with Cd had a clinically meaningful improvement in PFS compared with Bd regardless of baseline cytogenetic risk status. Higher response rates, greater depth of response and longer DOR were also reported with Cd vs Bd. Cd had a favorable benefit–risk profile in patients with high-risk relapsed MM.

Session topic: E-poster
Keyword(s): Cytogenetics, Multiple myeloma, Proteasome inhibitor, Relapse
Type: Eposter Presentation
Background
In a previous study, single-agent carfilzomib demonstrated activity in patients with relapsed and refractory multiple myeloma with high-risk cytogenetic abnormalities. In the phase 3 study (NCT01568866; N=929 patients) carfilzomib plus dexamethasone (Cd) significantly improved progression-free survival (PFS) by 2-fold compared with bortezomib/dexamethasone (Bd) in patients with relapsed multiple myeloma (RMM) (18.7 months versus 9.4 months; hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.44–0.65; P<.0001).
Aims
A pre-planned subgroup analysis of efficacy and safety outcomes in patients treated with Cd vs Bd according to patients’ baseline cytogenetic risk status.
Methods
Adults with RMM who had received 1–3 prior lines of therapy received either carfilzomib (30-minute intravenous [IV] infusion on days 1, 2, 8, 9, 15, and 16 [20mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter]) and dexamethasone (20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle) (Cd) or bortezomib (1.3mg/m2 IV bolus or subcutaneous injection on days 1, 4, 8, and 11) and dexamethasone (20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle) (Bd). Treatment continued until disease progression, withdrawal of consent or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety. The high-risk group was defined (using fluorescence in situ hybridization analysis of baseline bone marrow samples) as those patients with the genetic subtypes t(4;14) or t(14;16) in ≥10% of screened plasma cells or deletion 17p in ≥20% of screened plasma cells; the standard-risk group consisted of patients without these genetic subtypes.
Results
Of the 929 patients, 464 were randomized to receive Cd and 465 to receive Bd. Baseline cytogenetic risk status was balanced between the treatment arms (high-risk: Cd, 20.9%; Bd, 24.3%; standard-risk: Cd, 61.2%; Bd, 62.6%; unknown: Cd, 17.9%; Bd, 13.1%). Efficacy and safety end points by baseline cytogenetic risk status are presented in the Table. In the high-risk group, median PFS was 8.8 months (95% CI: 6.9, 11.3) for Cd vs 6.0 months (95% CI: 4.9, 8.1) for Bd (HR: 0.646; 95% CI: 0.453, 0.921). In the standard-risk group, median PFS was not estimable (NE) for Cd (95% CI: 18.7, NE) vs 10.2 months (95% CI: 9.3, 12.2) for Bd (HR: 0.439; 95% CI: 0.333, 0.578). ORRs (≥partial response) in the high-risk group were 72.2% (Cd) vs 58.4% (Bd) and 79.2% (Cd) vs 66.0% (Bd) in the standard-risk group. Median DOR in the high-risk group was 10.2 months for Cd vs 8.3 months for Bd. Median DOR in the standard-risk group was NE for Cd vs 11.7 months for Bd. Grade ≥3 adverse events (AEs) were reported at higher rates with Cd vs Bd in both the high- and standard-risk groups (70.1% vs 63.1% and 73.9% vs 68.3%). Grade ≥2 PN occurred less frequently with Cd vs Bd regardless of cytogenetic risk status (high-risk group: 3.1% vs 35.1%; odds ratio: 0.059; 95% CI: 0.018, 0.198 and standard-risk group: 6.4% vs 33.4%; odds ratio: 0.135; 95% CI: 0.079, 0.231).
Conclusion
Patients treated with Cd had a clinically meaningful improvement in PFS compared with Bd regardless of baseline cytogenetic risk status. Higher response rates, greater depth of response and longer DOR were also reported with Cd vs Bd. Cd had a favorable benefit–risk profile in patients with high-risk relapsed MM.

Session topic: E-poster
Keyword(s): Cytogenetics, Multiple myeloma, Proteasome inhibitor, Relapse
{{ help_message }}
{{filter}}


