CARFILZOMIB AND DEXAMETHASONE VERSUS BORTEZOMIB AND DEXAMETHASONE: SUBGROUP ANALYSIS OF THE PHASE 3 ENDEAVOR STUDY TO EVALUATE THE IMPACT OF PRIOR TREATMENT ON PATIENTS WITH RELAPSED MULTIPLE MYELOMA
(Abstract release date: 05/19/16)
EHA Library. Moreau P. 06/09/16; 132815; E1266

Prof. Philippe Moreau
Contributions
Contributions
Abstract
Abstract: E1266
Type: Eposter Presentation
Background
In a randomized phase 3 study (ENDEAVOR; NCT01568866), carfilzomib and dexamethasone (Cd) significantly improved median progression-free survival (PFS) versus bortezomib (BTZ) and dexamethasone (Bd) (18.7 vs 9.4 months; hazard ratio [HR] 0.53; 95% confidence interval [CI]: 0.44, 0.65; p<0.0001) in 929 patients with relapsed multiple myeloma (RMM).
Aims
This subgroup analysis evaluated treatment with Cd versus Bd in patients after first relapse versus ≥2 prior lines of therapy as well as the effect of previous exposure to BTZ or lenalidomide (LEN).
Methods
Adult patients with RMM who had received 1-3 prior lines of therapy were randomized 1:1 to Cd or Bd. Patients in the Cd arm received carfilzomib (30-min intravenous [IV] infusion) on days 1, 2, 8, 9, 15, and 16 (20mg/m2 on days 1 and 2 of cycle 1; 56mg/m2 thereafter) and dexamethasone 20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle. Patients in the Bd arm received BTZ 1.3mg/m2 (IV or subcutaneous) on days 1, 4, 8, and 11 and dexamethasone 20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle. Treatment was continued until disease progression or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR; ≥partial response), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety.
Results
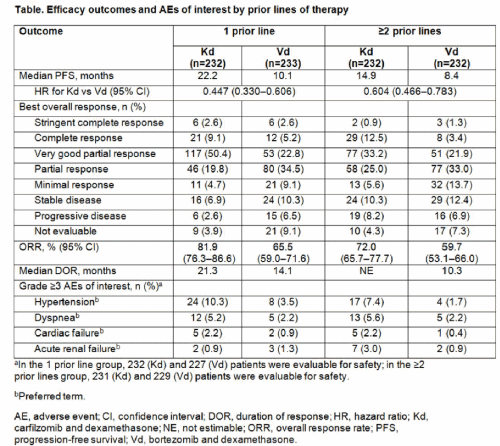
Efficacy outcomes by prior lines of therapy are shown in the Table. Median PFS for Cd vs Bd was 15.6 months vs 8.1 months, respectively (HR: 0.56; 95% CI: 0.44, 0.73) for patients with prior BTZ exposure and NE vs 11.2 months, respectively (HR: 0.48; 95% CI: 0.36, 0.66) for patients without prior BTZ exposure. Median PFS for Cd vs Bd was 12.9 months vs 7.3 months, respectively (HR: 0.69; 95% CI: 0.52, 0.92) for patients with prior LEN exposure and 22.2 months vs 10.2 months, respectively (HR: 0.43; 95% CI: 0.32, 0.56) for patients without prior LEN exposure. ORRs for Cd vs Bd were 71.2% vs 60.3% (OR: 1.63; 95% CI: 1.12, 2.36) in patients with prior BTZ exposure, 83.6% vs 65.3% (OR: 2.72; 95% CI: 1.72, 4.31) in patients without prior BTZ exposure, 70.1% vs 59.3% (OR: 1.60; 95% CI: 1.03, 2.49) in patients with prior LEN exposure and 81.2% vs 64.6% (OR: 2.37; 95% CI: 1.62, 3.47) in patients without prior LEN exposure. Grade ≥3 adverse events (AEs) were reported in 69.8% (Cd) and 63.9% (Bd) of patients with 1 prior line and 76.6% (Cd) and 69.9% (Bd) of patients with ≥2 prior lines. Grade ≥3 hypertension, dyspnea, and cardiac failure were more common with Cd vs Bd. The rate of grade ≥2 PN was lower with Cd vs Bd in patients with 1 prior line (6.5% vs 30.0%; OR: 0.16; 95% CI: 0.09, 0.29) and ≥2 prior lines (5.6% vs 34.1%; OR: 0.12; 95% CI: 0.06, 0.22).
Conclusion
A clinically meaningful improvement in PFS was seen for patients with RMM who were treated with Cd compared with Bd, regardless of the number of prior lines of treatment. The improvement was greatest for those who had received 1 prior line, where median PFS was over 1 year longer in patients treated with Cd vs Bd. PFS benefit with Cd vs Bd was also maintained regardless of prior exposure to BTZ and LEN. A higher ORR was also observed with Cd vs Bd across all subgroups. Cd had an acceptable benefit-risk profile in this study.
Session topic: E-poster
Keyword(s): Multiple myeloma, Proteasome inhibitor, Relapse
Type: Eposter Presentation
Background
In a randomized phase 3 study (ENDEAVOR; NCT01568866), carfilzomib and dexamethasone (Cd) significantly improved median progression-free survival (PFS) versus bortezomib (BTZ) and dexamethasone (Bd) (18.7 vs 9.4 months; hazard ratio [HR] 0.53; 95% confidence interval [CI]: 0.44, 0.65; p<0.0001) in 929 patients with relapsed multiple myeloma (RMM).
Aims
This subgroup analysis evaluated treatment with Cd versus Bd in patients after first relapse versus ≥2 prior lines of therapy as well as the effect of previous exposure to BTZ or lenalidomide (LEN).
Methods
Adult patients with RMM who had received 1-3 prior lines of therapy were randomized 1:1 to Cd or Bd. Patients in the Cd arm received carfilzomib (30-min intravenous [IV] infusion) on days 1, 2, 8, 9, 15, and 16 (20mg/m2 on days 1 and 2 of cycle 1; 56mg/m2 thereafter) and dexamethasone 20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle. Patients in the Bd arm received BTZ 1.3mg/m2 (IV or subcutaneous) on days 1, 4, 8, and 11 and dexamethasone 20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle. Treatment was continued until disease progression or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR; ≥partial response), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety.
Results
Efficacy outcomes by prior lines of therapy are shown in the Table. Median PFS for Cd vs Bd was 15.6 months vs 8.1 months, respectively (HR: 0.56; 95% CI: 0.44, 0.73) for patients with prior BTZ exposure and NE vs 11.2 months, respectively (HR: 0.48; 95% CI: 0.36, 0.66) for patients without prior BTZ exposure. Median PFS for Cd vs Bd was 12.9 months vs 7.3 months, respectively (HR: 0.69; 95% CI: 0.52, 0.92) for patients with prior LEN exposure and 22.2 months vs 10.2 months, respectively (HR: 0.43; 95% CI: 0.32, 0.56) for patients without prior LEN exposure. ORRs for Cd vs Bd were 71.2% vs 60.3% (OR: 1.63; 95% CI: 1.12, 2.36) in patients with prior BTZ exposure, 83.6% vs 65.3% (OR: 2.72; 95% CI: 1.72, 4.31) in patients without prior BTZ exposure, 70.1% vs 59.3% (OR: 1.60; 95% CI: 1.03, 2.49) in patients with prior LEN exposure and 81.2% vs 64.6% (OR: 2.37; 95% CI: 1.62, 3.47) in patients without prior LEN exposure. Grade ≥3 adverse events (AEs) were reported in 69.8% (Cd) and 63.9% (Bd) of patients with 1 prior line and 76.6% (Cd) and 69.9% (Bd) of patients with ≥2 prior lines. Grade ≥3 hypertension, dyspnea, and cardiac failure were more common with Cd vs Bd. The rate of grade ≥2 PN was lower with Cd vs Bd in patients with 1 prior line (6.5% vs 30.0%; OR: 0.16; 95% CI: 0.09, 0.29) and ≥2 prior lines (5.6% vs 34.1%; OR: 0.12; 95% CI: 0.06, 0.22).
Conclusion
A clinically meaningful improvement in PFS was seen for patients with RMM who were treated with Cd compared with Bd, regardless of the number of prior lines of treatment. The improvement was greatest for those who had received 1 prior line, where median PFS was over 1 year longer in patients treated with Cd vs Bd. PFS benefit with Cd vs Bd was also maintained regardless of prior exposure to BTZ and LEN. A higher ORR was also observed with Cd vs Bd across all subgroups. Cd had an acceptable benefit-risk profile in this study.

Session topic: E-poster
Keyword(s): Multiple myeloma, Proteasome inhibitor, Relapse
Abstract: E1266
Type: Eposter Presentation
Background
In a randomized phase 3 study (ENDEAVOR; NCT01568866), carfilzomib and dexamethasone (Cd) significantly improved median progression-free survival (PFS) versus bortezomib (BTZ) and dexamethasone (Bd) (18.7 vs 9.4 months; hazard ratio [HR] 0.53; 95% confidence interval [CI]: 0.44, 0.65; p<0.0001) in 929 patients with relapsed multiple myeloma (RMM).
Aims
This subgroup analysis evaluated treatment with Cd versus Bd in patients after first relapse versus ≥2 prior lines of therapy as well as the effect of previous exposure to BTZ or lenalidomide (LEN).
Methods
Adult patients with RMM who had received 1-3 prior lines of therapy were randomized 1:1 to Cd or Bd. Patients in the Cd arm received carfilzomib (30-min intravenous [IV] infusion) on days 1, 2, 8, 9, 15, and 16 (20mg/m2 on days 1 and 2 of cycle 1; 56mg/m2 thereafter) and dexamethasone 20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle. Patients in the Bd arm received BTZ 1.3mg/m2 (IV or subcutaneous) on days 1, 4, 8, and 11 and dexamethasone 20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle. Treatment was continued until disease progression or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR; ≥partial response), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety.
Results
Efficacy outcomes by prior lines of therapy are shown in the Table. Median PFS for Cd vs Bd was 15.6 months vs 8.1 months, respectively (HR: 0.56; 95% CI: 0.44, 0.73) for patients with prior BTZ exposure and NE vs 11.2 months, respectively (HR: 0.48; 95% CI: 0.36, 0.66) for patients without prior BTZ exposure. Median PFS for Cd vs Bd was 12.9 months vs 7.3 months, respectively (HR: 0.69; 95% CI: 0.52, 0.92) for patients with prior LEN exposure and 22.2 months vs 10.2 months, respectively (HR: 0.43; 95% CI: 0.32, 0.56) for patients without prior LEN exposure. ORRs for Cd vs Bd were 71.2% vs 60.3% (OR: 1.63; 95% CI: 1.12, 2.36) in patients with prior BTZ exposure, 83.6% vs 65.3% (OR: 2.72; 95% CI: 1.72, 4.31) in patients without prior BTZ exposure, 70.1% vs 59.3% (OR: 1.60; 95% CI: 1.03, 2.49) in patients with prior LEN exposure and 81.2% vs 64.6% (OR: 2.37; 95% CI: 1.62, 3.47) in patients without prior LEN exposure. Grade ≥3 adverse events (AEs) were reported in 69.8% (Cd) and 63.9% (Bd) of patients with 1 prior line and 76.6% (Cd) and 69.9% (Bd) of patients with ≥2 prior lines. Grade ≥3 hypertension, dyspnea, and cardiac failure were more common with Cd vs Bd. The rate of grade ≥2 PN was lower with Cd vs Bd in patients with 1 prior line (6.5% vs 30.0%; OR: 0.16; 95% CI: 0.09, 0.29) and ≥2 prior lines (5.6% vs 34.1%; OR: 0.12; 95% CI: 0.06, 0.22).
Conclusion
A clinically meaningful improvement in PFS was seen for patients with RMM who were treated with Cd compared with Bd, regardless of the number of prior lines of treatment. The improvement was greatest for those who had received 1 prior line, where median PFS was over 1 year longer in patients treated with Cd vs Bd. PFS benefit with Cd vs Bd was also maintained regardless of prior exposure to BTZ and LEN. A higher ORR was also observed with Cd vs Bd across all subgroups. Cd had an acceptable benefit-risk profile in this study.

Session topic: E-poster
Keyword(s): Multiple myeloma, Proteasome inhibitor, Relapse
Type: Eposter Presentation
Background
In a randomized phase 3 study (ENDEAVOR; NCT01568866), carfilzomib and dexamethasone (Cd) significantly improved median progression-free survival (PFS) versus bortezomib (BTZ) and dexamethasone (Bd) (18.7 vs 9.4 months; hazard ratio [HR] 0.53; 95% confidence interval [CI]: 0.44, 0.65; p<0.0001) in 929 patients with relapsed multiple myeloma (RMM).
Aims
This subgroup analysis evaluated treatment with Cd versus Bd in patients after first relapse versus ≥2 prior lines of therapy as well as the effect of previous exposure to BTZ or lenalidomide (LEN).
Methods
Adult patients with RMM who had received 1-3 prior lines of therapy were randomized 1:1 to Cd or Bd. Patients in the Cd arm received carfilzomib (30-min intravenous [IV] infusion) on days 1, 2, 8, 9, 15, and 16 (20mg/m2 on days 1 and 2 of cycle 1; 56mg/m2 thereafter) and dexamethasone 20mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle. Patients in the Bd arm received BTZ 1.3mg/m2 (IV or subcutaneous) on days 1, 4, 8, and 11 and dexamethasone 20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle. Treatment was continued until disease progression or unacceptable toxicity. Primary end point was PFS, secondary end points included overall survival, overall response rate (ORR; ≥partial response), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN) and safety.
Results
Efficacy outcomes by prior lines of therapy are shown in the Table. Median PFS for Cd vs Bd was 15.6 months vs 8.1 months, respectively (HR: 0.56; 95% CI: 0.44, 0.73) for patients with prior BTZ exposure and NE vs 11.2 months, respectively (HR: 0.48; 95% CI: 0.36, 0.66) for patients without prior BTZ exposure. Median PFS for Cd vs Bd was 12.9 months vs 7.3 months, respectively (HR: 0.69; 95% CI: 0.52, 0.92) for patients with prior LEN exposure and 22.2 months vs 10.2 months, respectively (HR: 0.43; 95% CI: 0.32, 0.56) for patients without prior LEN exposure. ORRs for Cd vs Bd were 71.2% vs 60.3% (OR: 1.63; 95% CI: 1.12, 2.36) in patients with prior BTZ exposure, 83.6% vs 65.3% (OR: 2.72; 95% CI: 1.72, 4.31) in patients without prior BTZ exposure, 70.1% vs 59.3% (OR: 1.60; 95% CI: 1.03, 2.49) in patients with prior LEN exposure and 81.2% vs 64.6% (OR: 2.37; 95% CI: 1.62, 3.47) in patients without prior LEN exposure. Grade ≥3 adverse events (AEs) were reported in 69.8% (Cd) and 63.9% (Bd) of patients with 1 prior line and 76.6% (Cd) and 69.9% (Bd) of patients with ≥2 prior lines. Grade ≥3 hypertension, dyspnea, and cardiac failure were more common with Cd vs Bd. The rate of grade ≥2 PN was lower with Cd vs Bd in patients with 1 prior line (6.5% vs 30.0%; OR: 0.16; 95% CI: 0.09, 0.29) and ≥2 prior lines (5.6% vs 34.1%; OR: 0.12; 95% CI: 0.06, 0.22).
Conclusion
A clinically meaningful improvement in PFS was seen for patients with RMM who were treated with Cd compared with Bd, regardless of the number of prior lines of treatment. The improvement was greatest for those who had received 1 prior line, where median PFS was over 1 year longer in patients treated with Cd vs Bd. PFS benefit with Cd vs Bd was also maintained regardless of prior exposure to BTZ and LEN. A higher ORR was also observed with Cd vs Bd across all subgroups. Cd had an acceptable benefit-risk profile in this study.

Session topic: E-poster
Keyword(s): Multiple myeloma, Proteasome inhibitor, Relapse
{{ help_message }}
{{filter}}


