The Swedish Multiple Myeloma Registry: Median survival exceeds 7 years in patients 65 years and younger, population-based data on 3,876 multiple myeloma patients diagnosed 2008-2013
(Abstract release date: 05/19/16)
EHA Library. Hveding Blimark C. 06/09/16; 132814; E1265
Disclosure(s): Advisary Board: Takeda, Amgen, Janssen
Consultant Honorary: Amgen, Grifols

Dr. Cecilie Hveding Blimark
Contributions
Contributions
Abstract
Abstract: E1265
Type: Eposter Presentation
Background
The Swedish Multiple Myeloma Register (SMMR) is a prospective observational register designed to document real-world management and outcomes in newly diagnosed multiple myeloma (MM), and was designed to improve the quality of the management of MM patients in Sweden
Aims
With high representation and good data quality we can present survival data on a whole MM population.
Methods
SMMR, initiated in 2008, comprises data on all patients diagnosed with MM, plasmocytoma, and plasma cell leukemia treated in Sweden. Report sheet are sent to all clinicians diagnosing MM. Another request is sent to the clinician one year after diagnosis, requesting information on initial treatment and complications. Through linkage with the Register of Total Population, we collected information on vital status until August 6, 2015. This report contains data on patients reported to SMMR between 2008 and 2013 and follow-up data from the first year on patients with symptomatic MM 2008-2012, with a follow-up till August 6, 2015, with focus on survival at 1, 3 and 5 years. Analyses of incidence, characteristics at baseline, proportion of patients given intensive treatment, obtaining very good partial remission rate (VGPR) and overall survival (OS) were estimated. Survival data was analysed with respect to age and ISS stage at diagnosis.
Results
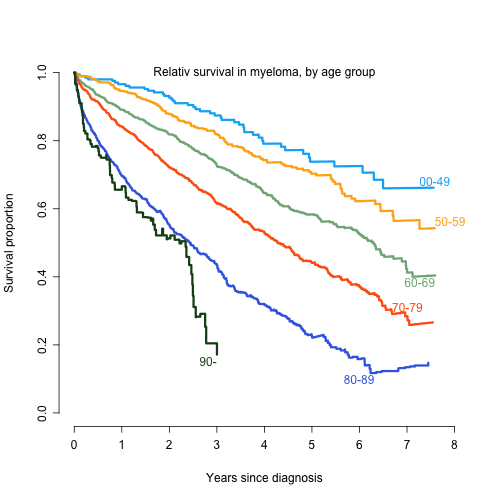
The SMMR has a current coverage of 98 % compared to the Swedish Cancer Register on patients 2008-2013, to which reporting is obliged by law. Clinical data at baseline was available for 3876 patients and one year follow-up data was available on 2284 symptomatic MM patients (92 % of all symptomatic MM cases initially reported to the register), from 70 different centers in Sweden. The median time of follow-up was 4.6 years. The age adjusted incidence was 6.2 MM cases per 100 000 inhabitants and year. The median age was 71 years, 70 years for men, and 73 years for women. Two third of patients were 65 years or older. Of the reported patients, 29% were in ISS stage I, 42% in stage II, and 29% in stage III. Overall, 80 % of patients 65 years or younger received ASCT and 4% of patients above 65 years. In patients 66-70 years, ASCT was performed in 19%. In the year 2012, 67% of patients received one of the new drugs (thalidomide, bortezomib, or lenalidomide) as induction, (87% of patients <66 and 60% >65), an improvement compared to earlier years. The response grade >VGPR increased from 36% to 46% in the study period. The 1-, 3- and 5-year relative survival was 92%, 79%, and 66% for patients 65 years and younger and 79%, 56%, and 39% for the patients > 65 years, respectively. The median relative survival was 7.3 and 3.7 years for patients < 66 years and > 65 years, respectively. Early death (< 1year after diagnosis) was observed in 19% of patients. The relative median survival according to ISS stage was 3.2 years and 6.0 years for stages III and II, while not reached for stage I. Patients with no reported stage had the same survival as stage III patients.
Conclusion
The SMMR is an instrument for increased quality in the management of MM in Sweden. This report shows encouraging survival data on the MM population of Sweden. There was a significant difference in survival for younger and older patients that cannot be explained by age alone. The 19 % early death rate (< 1 year survival) can be compared to 48 % in the period 1964 - 1968 (Swedish Cancer register data ) and illustrates the enormous development in the care of MM patients in Sweden during the last 50 years.
Session topic: E-poster
Keyword(s): Incidence, Multiple myeloma, Survival
Type: Eposter Presentation
Background
The Swedish Multiple Myeloma Register (SMMR) is a prospective observational register designed to document real-world management and outcomes in newly diagnosed multiple myeloma (MM), and was designed to improve the quality of the management of MM patients in Sweden
Aims
With high representation and good data quality we can present survival data on a whole MM population.
Methods
SMMR, initiated in 2008, comprises data on all patients diagnosed with MM, plasmocytoma, and plasma cell leukemia treated in Sweden. Report sheet are sent to all clinicians diagnosing MM. Another request is sent to the clinician one year after diagnosis, requesting information on initial treatment and complications. Through linkage with the Register of Total Population, we collected information on vital status until August 6, 2015. This report contains data on patients reported to SMMR between 2008 and 2013 and follow-up data from the first year on patients with symptomatic MM 2008-2012, with a follow-up till August 6, 2015, with focus on survival at 1, 3 and 5 years. Analyses of incidence, characteristics at baseline, proportion of patients given intensive treatment, obtaining very good partial remission rate (VGPR) and overall survival (OS) were estimated. Survival data was analysed with respect to age and ISS stage at diagnosis.
Results
The SMMR has a current coverage of 98 % compared to the Swedish Cancer Register on patients 2008-2013, to which reporting is obliged by law. Clinical data at baseline was available for 3876 patients and one year follow-up data was available on 2284 symptomatic MM patients (92 % of all symptomatic MM cases initially reported to the register), from 70 different centers in Sweden. The median time of follow-up was 4.6 years. The age adjusted incidence was 6.2 MM cases per 100 000 inhabitants and year. The median age was 71 years, 70 years for men, and 73 years for women. Two third of patients were 65 years or older. Of the reported patients, 29% were in ISS stage I, 42% in stage II, and 29% in stage III. Overall, 80 % of patients 65 years or younger received ASCT and 4% of patients above 65 years. In patients 66-70 years, ASCT was performed in 19%. In the year 2012, 67% of patients received one of the new drugs (thalidomide, bortezomib, or lenalidomide) as induction, (87% of patients <66 and 60% >65), an improvement compared to earlier years. The response grade >VGPR increased from 36% to 46% in the study period. The 1-, 3- and 5-year relative survival was 92%, 79%, and 66% for patients 65 years and younger and 79%, 56%, and 39% for the patients > 65 years, respectively. The median relative survival was 7.3 and 3.7 years for patients < 66 years and > 65 years, respectively. Early death (< 1year after diagnosis) was observed in 19% of patients. The relative median survival according to ISS stage was 3.2 years and 6.0 years for stages III and II, while not reached for stage I. Patients with no reported stage had the same survival as stage III patients.
Conclusion
The SMMR is an instrument for increased quality in the management of MM in Sweden. This report shows encouraging survival data on the MM population of Sweden. There was a significant difference in survival for younger and older patients that cannot be explained by age alone. The 19 % early death rate (< 1 year survival) can be compared to 48 % in the period 1964 - 1968 (Swedish Cancer register data ) and illustrates the enormous development in the care of MM patients in Sweden during the last 50 years.

Session topic: E-poster
Keyword(s): Incidence, Multiple myeloma, Survival
Abstract: E1265
Type: Eposter Presentation
Background
The Swedish Multiple Myeloma Register (SMMR) is a prospective observational register designed to document real-world management and outcomes in newly diagnosed multiple myeloma (MM), and was designed to improve the quality of the management of MM patients in Sweden
Aims
With high representation and good data quality we can present survival data on a whole MM population.
Methods
SMMR, initiated in 2008, comprises data on all patients diagnosed with MM, plasmocytoma, and plasma cell leukemia treated in Sweden. Report sheet are sent to all clinicians diagnosing MM. Another request is sent to the clinician one year after diagnosis, requesting information on initial treatment and complications. Through linkage with the Register of Total Population, we collected information on vital status until August 6, 2015. This report contains data on patients reported to SMMR between 2008 and 2013 and follow-up data from the first year on patients with symptomatic MM 2008-2012, with a follow-up till August 6, 2015, with focus on survival at 1, 3 and 5 years. Analyses of incidence, characteristics at baseline, proportion of patients given intensive treatment, obtaining very good partial remission rate (VGPR) and overall survival (OS) were estimated. Survival data was analysed with respect to age and ISS stage at diagnosis.
Results
The SMMR has a current coverage of 98 % compared to the Swedish Cancer Register on patients 2008-2013, to which reporting is obliged by law. Clinical data at baseline was available for 3876 patients and one year follow-up data was available on 2284 symptomatic MM patients (92 % of all symptomatic MM cases initially reported to the register), from 70 different centers in Sweden. The median time of follow-up was 4.6 years. The age adjusted incidence was 6.2 MM cases per 100 000 inhabitants and year. The median age was 71 years, 70 years for men, and 73 years for women. Two third of patients were 65 years or older. Of the reported patients, 29% were in ISS stage I, 42% in stage II, and 29% in stage III. Overall, 80 % of patients 65 years or younger received ASCT and 4% of patients above 65 years. In patients 66-70 years, ASCT was performed in 19%. In the year 2012, 67% of patients received one of the new drugs (thalidomide, bortezomib, or lenalidomide) as induction, (87% of patients <66 and 60% >65), an improvement compared to earlier years. The response grade >VGPR increased from 36% to 46% in the study period. The 1-, 3- and 5-year relative survival was 92%, 79%, and 66% for patients 65 years and younger and 79%, 56%, and 39% for the patients > 65 years, respectively. The median relative survival was 7.3 and 3.7 years for patients < 66 years and > 65 years, respectively. Early death (< 1year after diagnosis) was observed in 19% of patients. The relative median survival according to ISS stage was 3.2 years and 6.0 years for stages III and II, while not reached for stage I. Patients with no reported stage had the same survival as stage III patients.
Conclusion
The SMMR is an instrument for increased quality in the management of MM in Sweden. This report shows encouraging survival data on the MM population of Sweden. There was a significant difference in survival for younger and older patients that cannot be explained by age alone. The 19 % early death rate (< 1 year survival) can be compared to 48 % in the period 1964 - 1968 (Swedish Cancer register data ) and illustrates the enormous development in the care of MM patients in Sweden during the last 50 years.

Session topic: E-poster
Keyword(s): Incidence, Multiple myeloma, Survival
Type: Eposter Presentation
Background
The Swedish Multiple Myeloma Register (SMMR) is a prospective observational register designed to document real-world management and outcomes in newly diagnosed multiple myeloma (MM), and was designed to improve the quality of the management of MM patients in Sweden
Aims
With high representation and good data quality we can present survival data on a whole MM population.
Methods
SMMR, initiated in 2008, comprises data on all patients diagnosed with MM, plasmocytoma, and plasma cell leukemia treated in Sweden. Report sheet are sent to all clinicians diagnosing MM. Another request is sent to the clinician one year after diagnosis, requesting information on initial treatment and complications. Through linkage with the Register of Total Population, we collected information on vital status until August 6, 2015. This report contains data on patients reported to SMMR between 2008 and 2013 and follow-up data from the first year on patients with symptomatic MM 2008-2012, with a follow-up till August 6, 2015, with focus on survival at 1, 3 and 5 years. Analyses of incidence, characteristics at baseline, proportion of patients given intensive treatment, obtaining very good partial remission rate (VGPR) and overall survival (OS) were estimated. Survival data was analysed with respect to age and ISS stage at diagnosis.
Results
The SMMR has a current coverage of 98 % compared to the Swedish Cancer Register on patients 2008-2013, to which reporting is obliged by law. Clinical data at baseline was available for 3876 patients and one year follow-up data was available on 2284 symptomatic MM patients (92 % of all symptomatic MM cases initially reported to the register), from 70 different centers in Sweden. The median time of follow-up was 4.6 years. The age adjusted incidence was 6.2 MM cases per 100 000 inhabitants and year. The median age was 71 years, 70 years for men, and 73 years for women. Two third of patients were 65 years or older. Of the reported patients, 29% were in ISS stage I, 42% in stage II, and 29% in stage III. Overall, 80 % of patients 65 years or younger received ASCT and 4% of patients above 65 years. In patients 66-70 years, ASCT was performed in 19%. In the year 2012, 67% of patients received one of the new drugs (thalidomide, bortezomib, or lenalidomide) as induction, (87% of patients <66 and 60% >65), an improvement compared to earlier years. The response grade >VGPR increased from 36% to 46% in the study period. The 1-, 3- and 5-year relative survival was 92%, 79%, and 66% for patients 65 years and younger and 79%, 56%, and 39% for the patients > 65 years, respectively. The median relative survival was 7.3 and 3.7 years for patients < 66 years and > 65 years, respectively. Early death (< 1year after diagnosis) was observed in 19% of patients. The relative median survival according to ISS stage was 3.2 years and 6.0 years for stages III and II, while not reached for stage I. Patients with no reported stage had the same survival as stage III patients.
Conclusion
The SMMR is an instrument for increased quality in the management of MM in Sweden. This report shows encouraging survival data on the MM population of Sweden. There was a significant difference in survival for younger and older patients that cannot be explained by age alone. The 19 % early death rate (< 1 year survival) can be compared to 48 % in the period 1964 - 1968 (Swedish Cancer register data ) and illustrates the enormous development in the care of MM patients in Sweden during the last 50 years.

Session topic: E-poster
Keyword(s): Incidence, Multiple myeloma, Survival
{{ help_message }}
{{filter}}


