EXPLORING THE BONE MARROW NICHE IN MULTIPLE MYELOMA USING AN ADHESION-INDEPENDENT THREE-DIMENSIONAL CO-CULTURE MODEL
(Abstract release date: 05/19/16)
EHA Library. Waldschmidt J. 06/09/16; 132791; E1242

Dr. Johannes Moritz Waldschmidt
Contributions
Contributions
Abstract
Abstract: E1242
Type: Eposter Presentation
Background
The interaction between malignant plasma cells and their bone marrow (BM) niche is crucial for MM pathogenesis. The BM niche provides a specific tumor microenvironment which leads to quiescence, drug resistance and ultimately progression of myeloma. We and others have previously observed that reduced sensitivity to bortezomib, pomalidomide or vorinostat in the presence of BM stromal cells (BMSCs) can be restored by adding the adhesion inhibitors AMD3100 and NOX-A12. However, little is known about the mechanisms of how the MM niche mediates protection.
Aims
Our focus was to develop a novel bone-derived in vitro 3D co-culture platform and assess the cellular composition and function of the BM niche by studying the effects of niche cell subfractions on MM growth.
Methods
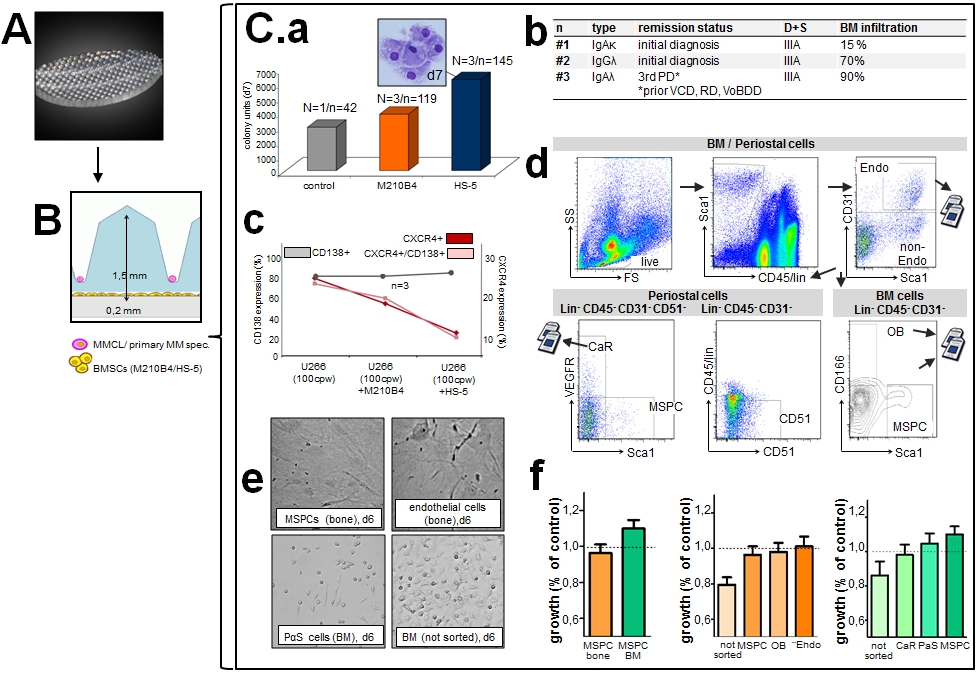
An adhesion-independent three-dimensional co-culture model was used to mimic the BM niche. This model consisted of an agarose matrix interlayer containing 100 microwells/cm² (Fig.1A). Each microwell was 1.5mm in depth and permeable for oxygen and cytokines, but not for BMSCs (Fig.1B). U266, RPMI-8226, OPM-2 and primary BM patient (pt) cells were utilized with and without (w/o) HS-5 vs. M210B4 stroma support (Fig.1C.a+b: stroma-support effect and pt characteristics). Analyses included trypan blue, Annexin/PI, MTT, FACS, cell cycle analyses and H2B-mCherry/cytochrome c-GFP assays (Udi, BJH 2013). To examine the effect of distinct BM niche cell populations on MM growth, niche cell subsets from C57BL6J mice were acquired, digested and FACS-sorted to collect cell subfractions of mesenchymal stem and progenitor cells (MSPC), endothelial cells, osteoblasts, premature CD146+ MSPCs (PαS) and CXCL12-abundant reticular cells (CaRs; Fig.1C.d).
Results
Human MM cell lines (MMCLs) and pt samples were cultured with 1, 10 or 100 cells/microwell, showing a growth advantage with vs w/o stroma support. Pt samples after 7 days (d) of culture benefitted from both murine M210-B4 and human HS-5 co-culture (Fig.1C.a). Expression of the chemokine CXCL12 and its corresponding receptor CXCR4 in U266 cells decreased after 7d with stroma support, thereby reflecting the dynamic regulation of the CXCL12-CXCR4 axis in vitro (Fig.1C.c). FACS-sorting of murine bone and BM cells led to valid subset separation, illustrated by a multipolar morphology of CD31+ endothelial cells and CD31-, CD45-, Sca1+ MSPCs with fibroblast-like bipolar appearance (Fig.1C.e), whereas PαS cells remained undifferentiated and positive for CD146. We observed that C57BL6J-derived murine cells differed in terms of their growth support for OPM-2 cells after 6d of co-culture: MSPCs from murine BM were more beneficial than those derived from murine bone (Fig.1C.f). BM-MSPCs also induced stronger support than premature MSPCs and CXCL12-abundant reticular cells, commonly considered as crucial mediators of adhesion in the murine niche. Current analyses focus on the generation of BM cell subsets from both MM pts and non-MM pts, thus overcoming the limitation of using C57BL6J mice.
Conclusion
We present a 3D culture model which reflects stromal protection for MM cells and may prove more reliable for ex-vivo drug screening and longitudinal studies. Assessment of the cellular composition of the MM niche in comparison to the normal BM microenvironment will help to better understand and selectively target stroma-mediated protection in MM.
Session topic: E-poster
Keyword(s): Bone marrow stroma, Drug resistance, Mesenchymal stem cell, Myeloma
Type: Eposter Presentation
Background
The interaction between malignant plasma cells and their bone marrow (BM) niche is crucial for MM pathogenesis. The BM niche provides a specific tumor microenvironment which leads to quiescence, drug resistance and ultimately progression of myeloma. We and others have previously observed that reduced sensitivity to bortezomib, pomalidomide or vorinostat in the presence of BM stromal cells (BMSCs) can be restored by adding the adhesion inhibitors AMD3100 and NOX-A12. However, little is known about the mechanisms of how the MM niche mediates protection.
Aims
Our focus was to develop a novel bone-derived in vitro 3D co-culture platform and assess the cellular composition and function of the BM niche by studying the effects of niche cell subfractions on MM growth.
Methods
An adhesion-independent three-dimensional co-culture model was used to mimic the BM niche. This model consisted of an agarose matrix interlayer containing 100 microwells/cm² (Fig.1A). Each microwell was 1.5mm in depth and permeable for oxygen and cytokines, but not for BMSCs (Fig.1B). U266, RPMI-8226, OPM-2 and primary BM patient (pt) cells were utilized with and without (w/o) HS-5 vs. M210B4 stroma support (Fig.1C.a+b: stroma-support effect and pt characteristics). Analyses included trypan blue, Annexin/PI, MTT, FACS, cell cycle analyses and H2B-mCherry/cytochrome c-GFP assays (Udi, BJH 2013). To examine the effect of distinct BM niche cell populations on MM growth, niche cell subsets from C57BL6J mice were acquired, digested and FACS-sorted to collect cell subfractions of mesenchymal stem and progenitor cells (MSPC), endothelial cells, osteoblasts, premature CD146+ MSPCs (PαS) and CXCL12-abundant reticular cells (CaRs; Fig.1C.d).
Results
Human MM cell lines (MMCLs) and pt samples were cultured with 1, 10 or 100 cells/microwell, showing a growth advantage with vs w/o stroma support. Pt samples after 7 days (d) of culture benefitted from both murine M210-B4 and human HS-5 co-culture (Fig.1C.a). Expression of the chemokine CXCL12 and its corresponding receptor CXCR4 in U266 cells decreased after 7d with stroma support, thereby reflecting the dynamic regulation of the CXCL12-CXCR4 axis in vitro (Fig.1C.c). FACS-sorting of murine bone and BM cells led to valid subset separation, illustrated by a multipolar morphology of CD31+ endothelial cells and CD31-, CD45-, Sca1+ MSPCs with fibroblast-like bipolar appearance (Fig.1C.e), whereas PαS cells remained undifferentiated and positive for CD146. We observed that C57BL6J-derived murine cells differed in terms of their growth support for OPM-2 cells after 6d of co-culture: MSPCs from murine BM were more beneficial than those derived from murine bone (Fig.1C.f). BM-MSPCs also induced stronger support than premature MSPCs and CXCL12-abundant reticular cells, commonly considered as crucial mediators of adhesion in the murine niche. Current analyses focus on the generation of BM cell subsets from both MM pts and non-MM pts, thus overcoming the limitation of using C57BL6J mice.
Conclusion
We present a 3D culture model which reflects stromal protection for MM cells and may prove more reliable for ex-vivo drug screening and longitudinal studies. Assessment of the cellular composition of the MM niche in comparison to the normal BM microenvironment will help to better understand and selectively target stroma-mediated protection in MM.

Session topic: E-poster
Keyword(s): Bone marrow stroma, Drug resistance, Mesenchymal stem cell, Myeloma
Abstract: E1242
Type: Eposter Presentation
Background
The interaction between malignant plasma cells and their bone marrow (BM) niche is crucial for MM pathogenesis. The BM niche provides a specific tumor microenvironment which leads to quiescence, drug resistance and ultimately progression of myeloma. We and others have previously observed that reduced sensitivity to bortezomib, pomalidomide or vorinostat in the presence of BM stromal cells (BMSCs) can be restored by adding the adhesion inhibitors AMD3100 and NOX-A12. However, little is known about the mechanisms of how the MM niche mediates protection.
Aims
Our focus was to develop a novel bone-derived in vitro 3D co-culture platform and assess the cellular composition and function of the BM niche by studying the effects of niche cell subfractions on MM growth.
Methods
An adhesion-independent three-dimensional co-culture model was used to mimic the BM niche. This model consisted of an agarose matrix interlayer containing 100 microwells/cm² (Fig.1A). Each microwell was 1.5mm in depth and permeable for oxygen and cytokines, but not for BMSCs (Fig.1B). U266, RPMI-8226, OPM-2 and primary BM patient (pt) cells were utilized with and without (w/o) HS-5 vs. M210B4 stroma support (Fig.1C.a+b: stroma-support effect and pt characteristics). Analyses included trypan blue, Annexin/PI, MTT, FACS, cell cycle analyses and H2B-mCherry/cytochrome c-GFP assays (Udi, BJH 2013). To examine the effect of distinct BM niche cell populations on MM growth, niche cell subsets from C57BL6J mice were acquired, digested and FACS-sorted to collect cell subfractions of mesenchymal stem and progenitor cells (MSPC), endothelial cells, osteoblasts, premature CD146+ MSPCs (PαS) and CXCL12-abundant reticular cells (CaRs; Fig.1C.d).
Results
Human MM cell lines (MMCLs) and pt samples were cultured with 1, 10 or 100 cells/microwell, showing a growth advantage with vs w/o stroma support. Pt samples after 7 days (d) of culture benefitted from both murine M210-B4 and human HS-5 co-culture (Fig.1C.a). Expression of the chemokine CXCL12 and its corresponding receptor CXCR4 in U266 cells decreased after 7d with stroma support, thereby reflecting the dynamic regulation of the CXCL12-CXCR4 axis in vitro (Fig.1C.c). FACS-sorting of murine bone and BM cells led to valid subset separation, illustrated by a multipolar morphology of CD31+ endothelial cells and CD31-, CD45-, Sca1+ MSPCs with fibroblast-like bipolar appearance (Fig.1C.e), whereas PαS cells remained undifferentiated and positive for CD146. We observed that C57BL6J-derived murine cells differed in terms of their growth support for OPM-2 cells after 6d of co-culture: MSPCs from murine BM were more beneficial than those derived from murine bone (Fig.1C.f). BM-MSPCs also induced stronger support than premature MSPCs and CXCL12-abundant reticular cells, commonly considered as crucial mediators of adhesion in the murine niche. Current analyses focus on the generation of BM cell subsets from both MM pts and non-MM pts, thus overcoming the limitation of using C57BL6J mice.
Conclusion
We present a 3D culture model which reflects stromal protection for MM cells and may prove more reliable for ex-vivo drug screening and longitudinal studies. Assessment of the cellular composition of the MM niche in comparison to the normal BM microenvironment will help to better understand and selectively target stroma-mediated protection in MM.

Session topic: E-poster
Keyword(s): Bone marrow stroma, Drug resistance, Mesenchymal stem cell, Myeloma
Type: Eposter Presentation
Background
The interaction between malignant plasma cells and their bone marrow (BM) niche is crucial for MM pathogenesis. The BM niche provides a specific tumor microenvironment which leads to quiescence, drug resistance and ultimately progression of myeloma. We and others have previously observed that reduced sensitivity to bortezomib, pomalidomide or vorinostat in the presence of BM stromal cells (BMSCs) can be restored by adding the adhesion inhibitors AMD3100 and NOX-A12. However, little is known about the mechanisms of how the MM niche mediates protection.
Aims
Our focus was to develop a novel bone-derived in vitro 3D co-culture platform and assess the cellular composition and function of the BM niche by studying the effects of niche cell subfractions on MM growth.
Methods
An adhesion-independent three-dimensional co-culture model was used to mimic the BM niche. This model consisted of an agarose matrix interlayer containing 100 microwells/cm² (Fig.1A). Each microwell was 1.5mm in depth and permeable for oxygen and cytokines, but not for BMSCs (Fig.1B). U266, RPMI-8226, OPM-2 and primary BM patient (pt) cells were utilized with and without (w/o) HS-5 vs. M210B4 stroma support (Fig.1C.a+b: stroma-support effect and pt characteristics). Analyses included trypan blue, Annexin/PI, MTT, FACS, cell cycle analyses and H2B-mCherry/cytochrome c-GFP assays (Udi, BJH 2013). To examine the effect of distinct BM niche cell populations on MM growth, niche cell subsets from C57BL6J mice were acquired, digested and FACS-sorted to collect cell subfractions of mesenchymal stem and progenitor cells (MSPC), endothelial cells, osteoblasts, premature CD146+ MSPCs (PαS) and CXCL12-abundant reticular cells (CaRs; Fig.1C.d).
Results
Human MM cell lines (MMCLs) and pt samples were cultured with 1, 10 or 100 cells/microwell, showing a growth advantage with vs w/o stroma support. Pt samples after 7 days (d) of culture benefitted from both murine M210-B4 and human HS-5 co-culture (Fig.1C.a). Expression of the chemokine CXCL12 and its corresponding receptor CXCR4 in U266 cells decreased after 7d with stroma support, thereby reflecting the dynamic regulation of the CXCL12-CXCR4 axis in vitro (Fig.1C.c). FACS-sorting of murine bone and BM cells led to valid subset separation, illustrated by a multipolar morphology of CD31+ endothelial cells and CD31-, CD45-, Sca1+ MSPCs with fibroblast-like bipolar appearance (Fig.1C.e), whereas PαS cells remained undifferentiated and positive for CD146. We observed that C57BL6J-derived murine cells differed in terms of their growth support for OPM-2 cells after 6d of co-culture: MSPCs from murine BM were more beneficial than those derived from murine bone (Fig.1C.f). BM-MSPCs also induced stronger support than premature MSPCs and CXCL12-abundant reticular cells, commonly considered as crucial mediators of adhesion in the murine niche. Current analyses focus on the generation of BM cell subsets from both MM pts and non-MM pts, thus overcoming the limitation of using C57BL6J mice.
Conclusion
We present a 3D culture model which reflects stromal protection for MM cells and may prove more reliable for ex-vivo drug screening and longitudinal studies. Assessment of the cellular composition of the MM niche in comparison to the normal BM microenvironment will help to better understand and selectively target stroma-mediated protection in MM.

Session topic: E-poster
Keyword(s): Bone marrow stroma, Drug resistance, Mesenchymal stem cell, Myeloma
{{ help_message }}
{{filter}}


