RHOU GTPASE: A NOVEL POTENTIAL TARGET TO DISRUPT MULTIPLE MYELOMA PLASMA CELL INTERACTION WITH PROTECTIVE BONE MARROW NICHES
(Abstract release date: 05/19/16)
EHA Library. Nunes S. 06/09/16; 132786; E1237

Ms. Sara Nunes
Contributions
Contributions
Abstract
Abstract: E1237
Type: Eposter Presentation
Background
Bone marrow stromal cell (BMSC)-produced soluble factors, like the IL-6 cytokine, may impinge on Multiple Myeloma (MM) intracellular signaling and cytoskeletal properties, protecting it from cytotoxic agents. Rho GTPases, in their active GTP-bound state, interact with effector proteins in order to control cytoskeleton remodeling, cell adhesion and polarization, and other essential processes for cell-cell interaction. The atypical Rho protein Wrch-1/RhoU displays spontaneous activation and is normally expressed at low levels in various tissues and organs. This protein might mediate the effects of the IL6R/STAT3 signaling in inducing filopodium formation and stress fiber dissolution, both critical steps in promoting cell motility. While typical Rho proteins (that share significant sequence homology with RhoU) as Cdc42 and Rac-1 have an established role in cancer, very little is known on RhoU in tumorigenesis, in particular in hematologic malignancies.
Aims
Since the IL6R/STAT3 signaling is of great importance in MM malignancy, we have endeavored to study RhoU expression in normal versus MM plasma cells. We also focused on understanding its localization and cellular function in these cells. Lastly, we aimed at unraveling the mechanisms through which RhoU expression is regulated in MM cells in the context of bone marrow (BM) microenvironment.
Methods
Malignant plasma cells were isolated from BM extracts or peripheral blood of MM patients using a magnetic CD138 positive selection kit. Normal B cells were purified using a magnetic human B cell isolation kit. mRNA expression was analyzed by qRT-PCR for MM cell lines, MM patients and normal B cells from healthy donors. Fluorescence microscopy was performed using Zeiss LSM 700 microscope and the following antibodies were used for staining: Phalloidin 594, rabbit anti-RhoU, goat anti nucleolin, anti-rabbit Alexa fluor 488, anti-goat Alexa fluor 594 and DAPI. STATTIC was used for STAT3 inhibition and the IL-6 cytokine was used to stimulate the IL-6R. Migration assays were set-up using 5mm transwells with standard controls, cell count was done by 3 minutes reads in FACS Canto.
Results
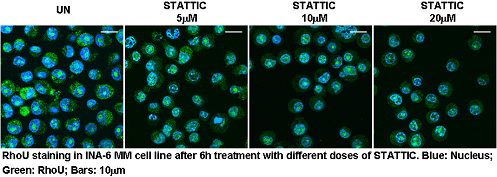
Here we provide data showing that RhoU is overexpressed in BMSC-dependent MM cells, but not in malignant plasma cells from the peripheral blood of Plasma Cell Leukemia (PCL) patients. We also demonstrate that its expression was positively modulated to the same extent by BMSC conditioned media and by IL-6, a major growth factor for MM cells. In MM cell lines, a blockade in STAT3 activation inhibited RhoU mRNA transcription in a dose dependent manner, and caused a clear impairment in migration/motility capacity of these cells. Interestingly, we observed an unexpected subcellular localization for this protein in the nucleolus of BMSC-dependent MM cells, which could be independent from STAT3 regulation.
Conclusion
RhoU GTPase is widely overexpressed in malignant plasma cells from BM extracts of MM patients but not in cells purified from the peripheral blood of PCL patients. Its expression is positively modulated by IL-6 stimulus through the activation of STAT3 transcription factor. We describe for the first time a localization of this G protein in the nucleolus of MM cells. These results put to light the pleiotropic features that RhoU could display in this malignancy. We firmly believe that RhoU might be important in MM pathogenesis and could become a suitable target to disrupt the MM plasma cell interaction with protective BM niches.
Session topic: E-poster
Keyword(s): Microenvironment, Multiple myeloma, Rho GTPase
Type: Eposter Presentation
Background
Bone marrow stromal cell (BMSC)-produced soluble factors, like the IL-6 cytokine, may impinge on Multiple Myeloma (MM) intracellular signaling and cytoskeletal properties, protecting it from cytotoxic agents. Rho GTPases, in their active GTP-bound state, interact with effector proteins in order to control cytoskeleton remodeling, cell adhesion and polarization, and other essential processes for cell-cell interaction. The atypical Rho protein Wrch-1/RhoU displays spontaneous activation and is normally expressed at low levels in various tissues and organs. This protein might mediate the effects of the IL6R/STAT3 signaling in inducing filopodium formation and stress fiber dissolution, both critical steps in promoting cell motility. While typical Rho proteins (that share significant sequence homology with RhoU) as Cdc42 and Rac-1 have an established role in cancer, very little is known on RhoU in tumorigenesis, in particular in hematologic malignancies.
Aims
Since the IL6R/STAT3 signaling is of great importance in MM malignancy, we have endeavored to study RhoU expression in normal versus MM plasma cells. We also focused on understanding its localization and cellular function in these cells. Lastly, we aimed at unraveling the mechanisms through which RhoU expression is regulated in MM cells in the context of bone marrow (BM) microenvironment.
Methods
Malignant plasma cells were isolated from BM extracts or peripheral blood of MM patients using a magnetic CD138 positive selection kit. Normal B cells were purified using a magnetic human B cell isolation kit. mRNA expression was analyzed by qRT-PCR for MM cell lines, MM patients and normal B cells from healthy donors. Fluorescence microscopy was performed using Zeiss LSM 700 microscope and the following antibodies were used for staining: Phalloidin 594, rabbit anti-RhoU, goat anti nucleolin, anti-rabbit Alexa fluor 488, anti-goat Alexa fluor 594 and DAPI. STATTIC was used for STAT3 inhibition and the IL-6 cytokine was used to stimulate the IL-6R. Migration assays were set-up using 5mm transwells with standard controls, cell count was done by 3 minutes reads in FACS Canto.
Results
Here we provide data showing that RhoU is overexpressed in BMSC-dependent MM cells, but not in malignant plasma cells from the peripheral blood of Plasma Cell Leukemia (PCL) patients. We also demonstrate that its expression was positively modulated to the same extent by BMSC conditioned media and by IL-6, a major growth factor for MM cells. In MM cell lines, a blockade in STAT3 activation inhibited RhoU mRNA transcription in a dose dependent manner, and caused a clear impairment in migration/motility capacity of these cells. Interestingly, we observed an unexpected subcellular localization for this protein in the nucleolus of BMSC-dependent MM cells, which could be independent from STAT3 regulation.
Conclusion
RhoU GTPase is widely overexpressed in malignant plasma cells from BM extracts of MM patients but not in cells purified from the peripheral blood of PCL patients. Its expression is positively modulated by IL-6 stimulus through the activation of STAT3 transcription factor. We describe for the first time a localization of this G protein in the nucleolus of MM cells. These results put to light the pleiotropic features that RhoU could display in this malignancy. We firmly believe that RhoU might be important in MM pathogenesis and could become a suitable target to disrupt the MM plasma cell interaction with protective BM niches.

Session topic: E-poster
Keyword(s): Microenvironment, Multiple myeloma, Rho GTPase
Abstract: E1237
Type: Eposter Presentation
Background
Bone marrow stromal cell (BMSC)-produced soluble factors, like the IL-6 cytokine, may impinge on Multiple Myeloma (MM) intracellular signaling and cytoskeletal properties, protecting it from cytotoxic agents. Rho GTPases, in their active GTP-bound state, interact with effector proteins in order to control cytoskeleton remodeling, cell adhesion and polarization, and other essential processes for cell-cell interaction. The atypical Rho protein Wrch-1/RhoU displays spontaneous activation and is normally expressed at low levels in various tissues and organs. This protein might mediate the effects of the IL6R/STAT3 signaling in inducing filopodium formation and stress fiber dissolution, both critical steps in promoting cell motility. While typical Rho proteins (that share significant sequence homology with RhoU) as Cdc42 and Rac-1 have an established role in cancer, very little is known on RhoU in tumorigenesis, in particular in hematologic malignancies.
Aims
Since the IL6R/STAT3 signaling is of great importance in MM malignancy, we have endeavored to study RhoU expression in normal versus MM plasma cells. We also focused on understanding its localization and cellular function in these cells. Lastly, we aimed at unraveling the mechanisms through which RhoU expression is regulated in MM cells in the context of bone marrow (BM) microenvironment.
Methods
Malignant plasma cells were isolated from BM extracts or peripheral blood of MM patients using a magnetic CD138 positive selection kit. Normal B cells were purified using a magnetic human B cell isolation kit. mRNA expression was analyzed by qRT-PCR for MM cell lines, MM patients and normal B cells from healthy donors. Fluorescence microscopy was performed using Zeiss LSM 700 microscope and the following antibodies were used for staining: Phalloidin 594, rabbit anti-RhoU, goat anti nucleolin, anti-rabbit Alexa fluor 488, anti-goat Alexa fluor 594 and DAPI. STATTIC was used for STAT3 inhibition and the IL-6 cytokine was used to stimulate the IL-6R. Migration assays were set-up using 5mm transwells with standard controls, cell count was done by 3 minutes reads in FACS Canto.
Results
Here we provide data showing that RhoU is overexpressed in BMSC-dependent MM cells, but not in malignant plasma cells from the peripheral blood of Plasma Cell Leukemia (PCL) patients. We also demonstrate that its expression was positively modulated to the same extent by BMSC conditioned media and by IL-6, a major growth factor for MM cells. In MM cell lines, a blockade in STAT3 activation inhibited RhoU mRNA transcription in a dose dependent manner, and caused a clear impairment in migration/motility capacity of these cells. Interestingly, we observed an unexpected subcellular localization for this protein in the nucleolus of BMSC-dependent MM cells, which could be independent from STAT3 regulation.
Conclusion
RhoU GTPase is widely overexpressed in malignant plasma cells from BM extracts of MM patients but not in cells purified from the peripheral blood of PCL patients. Its expression is positively modulated by IL-6 stimulus through the activation of STAT3 transcription factor. We describe for the first time a localization of this G protein in the nucleolus of MM cells. These results put to light the pleiotropic features that RhoU could display in this malignancy. We firmly believe that RhoU might be important in MM pathogenesis and could become a suitable target to disrupt the MM plasma cell interaction with protective BM niches.

Session topic: E-poster
Keyword(s): Microenvironment, Multiple myeloma, Rho GTPase
Type: Eposter Presentation
Background
Bone marrow stromal cell (BMSC)-produced soluble factors, like the IL-6 cytokine, may impinge on Multiple Myeloma (MM) intracellular signaling and cytoskeletal properties, protecting it from cytotoxic agents. Rho GTPases, in their active GTP-bound state, interact with effector proteins in order to control cytoskeleton remodeling, cell adhesion and polarization, and other essential processes for cell-cell interaction. The atypical Rho protein Wrch-1/RhoU displays spontaneous activation and is normally expressed at low levels in various tissues and organs. This protein might mediate the effects of the IL6R/STAT3 signaling in inducing filopodium formation and stress fiber dissolution, both critical steps in promoting cell motility. While typical Rho proteins (that share significant sequence homology with RhoU) as Cdc42 and Rac-1 have an established role in cancer, very little is known on RhoU in tumorigenesis, in particular in hematologic malignancies.
Aims
Since the IL6R/STAT3 signaling is of great importance in MM malignancy, we have endeavored to study RhoU expression in normal versus MM plasma cells. We also focused on understanding its localization and cellular function in these cells. Lastly, we aimed at unraveling the mechanisms through which RhoU expression is regulated in MM cells in the context of bone marrow (BM) microenvironment.
Methods
Malignant plasma cells were isolated from BM extracts or peripheral blood of MM patients using a magnetic CD138 positive selection kit. Normal B cells were purified using a magnetic human B cell isolation kit. mRNA expression was analyzed by qRT-PCR for MM cell lines, MM patients and normal B cells from healthy donors. Fluorescence microscopy was performed using Zeiss LSM 700 microscope and the following antibodies were used for staining: Phalloidin 594, rabbit anti-RhoU, goat anti nucleolin, anti-rabbit Alexa fluor 488, anti-goat Alexa fluor 594 and DAPI. STATTIC was used for STAT3 inhibition and the IL-6 cytokine was used to stimulate the IL-6R. Migration assays were set-up using 5mm transwells with standard controls, cell count was done by 3 minutes reads in FACS Canto.
Results
Here we provide data showing that RhoU is overexpressed in BMSC-dependent MM cells, but not in malignant plasma cells from the peripheral blood of Plasma Cell Leukemia (PCL) patients. We also demonstrate that its expression was positively modulated to the same extent by BMSC conditioned media and by IL-6, a major growth factor for MM cells. In MM cell lines, a blockade in STAT3 activation inhibited RhoU mRNA transcription in a dose dependent manner, and caused a clear impairment in migration/motility capacity of these cells. Interestingly, we observed an unexpected subcellular localization for this protein in the nucleolus of BMSC-dependent MM cells, which could be independent from STAT3 regulation.
Conclusion
RhoU GTPase is widely overexpressed in malignant plasma cells from BM extracts of MM patients but not in cells purified from the peripheral blood of PCL patients. Its expression is positively modulated by IL-6 stimulus through the activation of STAT3 transcription factor. We describe for the first time a localization of this G protein in the nucleolus of MM cells. These results put to light the pleiotropic features that RhoU could display in this malignancy. We firmly believe that RhoU might be important in MM pathogenesis and could become a suitable target to disrupt the MM plasma cell interaction with protective BM niches.

Session topic: E-poster
Keyword(s): Microenvironment, Multiple myeloma, Rho GTPase
{{ help_message }}
{{filter}}


