SPRESAS (SPANISH REGISTRY OF ERYTHROPOIETIC STIMULATING AGENTS STUDY) SUBANALYSIS: IMPACT OF COMORBIDITIES AND CENTER LEVEL ON RESPONSE TO ESAS IN LOWER RISK MDS
(Abstract release date: 05/19/16)
EHA Library. Davila Valls J. 06/09/16; 132781; E1232
Disclosure(s): I have nothing to disclose

Dr. Julio Davila Valls
Contributions
Contributions
Abstract
Abstract: E1232
Type: Eposter Presentation
Background
Myelodisplastic syndromes (MDS) are heterogeneous diseases in old patients. These patients show very often comorbidities that could decrease the overall survival and the quality of life. Erythropoietic-stimulating agents (ESAs) are the most used treatment for low risk MDS with anemia and are available in almost all centers from the smallest ones to the big reference centers. The impact of the presence of comorbidities and the size of center where the treatment is administered on treatment response has not been still analyzed.
Aims
The main endpoint was to evaluate the impact of comorbidities on the response to ESAs treatment. The secondary endpoint was to evaluate if the size of the hospital where this treatment is administered influences on it.
Methods
Data from 530 patients with low or intermediate-1 risk MDS (according to FAB and WHO criteria) who received treatments with ESAs were recorded in SPRESAS. 458 of these patients had data available at diagnosis of MDS regarding comorbidities. These were classified as relevant (diabetes mellitus, atrial fibrillation, thromboembolic disease and renal impairement) and less relevant (arterial hypertension, others). Data from the size of the center where the treatment was administered were available in all the patients. Centers were distributed in levels according to the number of beds available: centers above 1000 beds were considered “level 1” centers, between 500 and 1000 as “level 2” and below 500 beds as “level 3”. Response was evaluated according IWG 2006 criteria.
Results
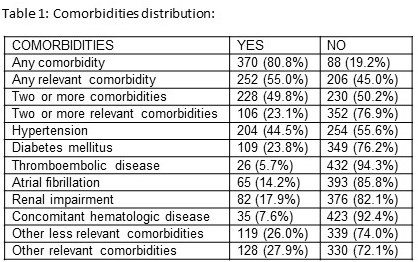
370 patients (80.8%) had at least one comorbidity, but when only relevant comorbidities were analyzed, only 252 patients (55.0%) presented at least one comorbidity (table 1). The presence of one comorbidity relevant or not did not show any impact on the overall response to ESAs (HR=1.441; p= 0.138 for one comorbidity; HR=1.278; p=0.223 for one relevant comorbidity). However, curiously, the presence of two or more comorbidities was associated with statistically significant better response to ESAs (68.9% response in patients with two or more comorbidities vs 64.2% in patients with less than two comorbidities; HR=3.014; p=0.004), the only difference between both groups was ferritine level while other variables with impact on response were similar in both groups (PB and BM blasts, Hb level, cytogenetics, transfusion dependency, IPSS and EPO level). Additionally, the presence of two or more relevant comorbidities was also associated with a better response (75.2% response in patients with two or more relevant comorbidities vs 64.0% in patients with less than two relevant comorbidities; HR= 4.126; p=0.001). These 2 groups were different regarding ferritine and EPO level. When the impact of the different comorbidities was studied, only hypertension showed influence on the overall response rate: patients with hypertension had statistically significant better response to ESAs (72.7%) than those who did not have hypertension (61.5%); p=0.015, both groups had similar characteristics regarding variables with impact on response. Regarding center size, 213 patients (40.2%) were treated in “level 1”; 174 (32.8%) in “level 2” and 143 (27.0%) in “level 3” centers. When the center level was analyzed we could verify that patients who were treated in the smallest “level 3” centers had a statistically significant higher overall response rate to ESAs (81.7%) when compared with “level 2” patients (66.0%) and “level 1” patients (52.8%), HR=3.995; p<0.001; there were less patients in transfusion dependence in “level 3” centers than in the others while other characteristics were similar in the 3 groups. No differences were observed regarding duration of ESAs response (p=0.098).
Conclusion
The present study shows that ESA treatment should not be avoided in patients with lower risk MDS with comorbidities, as response rates are similar to those of patients without. Patients treated in the smallest centers had the best response rates to ESA.
Session topic: E-poster
Keyword(s): Comorbidities, Myelodysplasia
Type: Eposter Presentation
Background
Myelodisplastic syndromes (MDS) are heterogeneous diseases in old patients. These patients show very often comorbidities that could decrease the overall survival and the quality of life. Erythropoietic-stimulating agents (ESAs) are the most used treatment for low risk MDS with anemia and are available in almost all centers from the smallest ones to the big reference centers. The impact of the presence of comorbidities and the size of center where the treatment is administered on treatment response has not been still analyzed.
Aims
The main endpoint was to evaluate the impact of comorbidities on the response to ESAs treatment. The secondary endpoint was to evaluate if the size of the hospital where this treatment is administered influences on it.
Methods
Data from 530 patients with low or intermediate-1 risk MDS (according to FAB and WHO criteria) who received treatments with ESAs were recorded in SPRESAS. 458 of these patients had data available at diagnosis of MDS regarding comorbidities. These were classified as relevant (diabetes mellitus, atrial fibrillation, thromboembolic disease and renal impairement) and less relevant (arterial hypertension, others). Data from the size of the center where the treatment was administered were available in all the patients. Centers were distributed in levels according to the number of beds available: centers above 1000 beds were considered “level 1” centers, between 500 and 1000 as “level 2” and below 500 beds as “level 3”. Response was evaluated according IWG 2006 criteria.
Results
370 patients (80.8%) had at least one comorbidity, but when only relevant comorbidities were analyzed, only 252 patients (55.0%) presented at least one comorbidity (table 1). The presence of one comorbidity relevant or not did not show any impact on the overall response to ESAs (HR=1.441; p= 0.138 for one comorbidity; HR=1.278; p=0.223 for one relevant comorbidity). However, curiously, the presence of two or more comorbidities was associated with statistically significant better response to ESAs (68.9% response in patients with two or more comorbidities vs 64.2% in patients with less than two comorbidities; HR=3.014; p=0.004), the only difference between both groups was ferritine level while other variables with impact on response were similar in both groups (PB and BM blasts, Hb level, cytogenetics, transfusion dependency, IPSS and EPO level). Additionally, the presence of two or more relevant comorbidities was also associated with a better response (75.2% response in patients with two or more relevant comorbidities vs 64.0% in patients with less than two relevant comorbidities; HR= 4.126; p=0.001). These 2 groups were different regarding ferritine and EPO level. When the impact of the different comorbidities was studied, only hypertension showed influence on the overall response rate: patients with hypertension had statistically significant better response to ESAs (72.7%) than those who did not have hypertension (61.5%); p=0.015, both groups had similar characteristics regarding variables with impact on response. Regarding center size, 213 patients (40.2%) were treated in “level 1”; 174 (32.8%) in “level 2” and 143 (27.0%) in “level 3” centers. When the center level was analyzed we could verify that patients who were treated in the smallest “level 3” centers had a statistically significant higher overall response rate to ESAs (81.7%) when compared with “level 2” patients (66.0%) and “level 1” patients (52.8%), HR=3.995; p<0.001; there were less patients in transfusion dependence in “level 3” centers than in the others while other characteristics were similar in the 3 groups. No differences were observed regarding duration of ESAs response (p=0.098).
Conclusion
The present study shows that ESA treatment should not be avoided in patients with lower risk MDS with comorbidities, as response rates are similar to those of patients without. Patients treated in the smallest centers had the best response rates to ESA.

Session topic: E-poster
Keyword(s): Comorbidities, Myelodysplasia
Abstract: E1232
Type: Eposter Presentation
Background
Myelodisplastic syndromes (MDS) are heterogeneous diseases in old patients. These patients show very often comorbidities that could decrease the overall survival and the quality of life. Erythropoietic-stimulating agents (ESAs) are the most used treatment for low risk MDS with anemia and are available in almost all centers from the smallest ones to the big reference centers. The impact of the presence of comorbidities and the size of center where the treatment is administered on treatment response has not been still analyzed.
Aims
The main endpoint was to evaluate the impact of comorbidities on the response to ESAs treatment. The secondary endpoint was to evaluate if the size of the hospital where this treatment is administered influences on it.
Methods
Data from 530 patients with low or intermediate-1 risk MDS (according to FAB and WHO criteria) who received treatments with ESAs were recorded in SPRESAS. 458 of these patients had data available at diagnosis of MDS regarding comorbidities. These were classified as relevant (diabetes mellitus, atrial fibrillation, thromboembolic disease and renal impairement) and less relevant (arterial hypertension, others). Data from the size of the center where the treatment was administered were available in all the patients. Centers were distributed in levels according to the number of beds available: centers above 1000 beds were considered “level 1” centers, between 500 and 1000 as “level 2” and below 500 beds as “level 3”. Response was evaluated according IWG 2006 criteria.
Results
370 patients (80.8%) had at least one comorbidity, but when only relevant comorbidities were analyzed, only 252 patients (55.0%) presented at least one comorbidity (table 1). The presence of one comorbidity relevant or not did not show any impact on the overall response to ESAs (HR=1.441; p= 0.138 for one comorbidity; HR=1.278; p=0.223 for one relevant comorbidity). However, curiously, the presence of two or more comorbidities was associated with statistically significant better response to ESAs (68.9% response in patients with two or more comorbidities vs 64.2% in patients with less than two comorbidities; HR=3.014; p=0.004), the only difference between both groups was ferritine level while other variables with impact on response were similar in both groups (PB and BM blasts, Hb level, cytogenetics, transfusion dependency, IPSS and EPO level). Additionally, the presence of two or more relevant comorbidities was also associated with a better response (75.2% response in patients with two or more relevant comorbidities vs 64.0% in patients with less than two relevant comorbidities; HR= 4.126; p=0.001). These 2 groups were different regarding ferritine and EPO level. When the impact of the different comorbidities was studied, only hypertension showed influence on the overall response rate: patients with hypertension had statistically significant better response to ESAs (72.7%) than those who did not have hypertension (61.5%); p=0.015, both groups had similar characteristics regarding variables with impact on response. Regarding center size, 213 patients (40.2%) were treated in “level 1”; 174 (32.8%) in “level 2” and 143 (27.0%) in “level 3” centers. When the center level was analyzed we could verify that patients who were treated in the smallest “level 3” centers had a statistically significant higher overall response rate to ESAs (81.7%) when compared with “level 2” patients (66.0%) and “level 1” patients (52.8%), HR=3.995; p<0.001; there were less patients in transfusion dependence in “level 3” centers than in the others while other characteristics were similar in the 3 groups. No differences were observed regarding duration of ESAs response (p=0.098).
Conclusion
The present study shows that ESA treatment should not be avoided in patients with lower risk MDS with comorbidities, as response rates are similar to those of patients without. Patients treated in the smallest centers had the best response rates to ESA.

Session topic: E-poster
Keyword(s): Comorbidities, Myelodysplasia
Type: Eposter Presentation
Background
Myelodisplastic syndromes (MDS) are heterogeneous diseases in old patients. These patients show very often comorbidities that could decrease the overall survival and the quality of life. Erythropoietic-stimulating agents (ESAs) are the most used treatment for low risk MDS with anemia and are available in almost all centers from the smallest ones to the big reference centers. The impact of the presence of comorbidities and the size of center where the treatment is administered on treatment response has not been still analyzed.
Aims
The main endpoint was to evaluate the impact of comorbidities on the response to ESAs treatment. The secondary endpoint was to evaluate if the size of the hospital where this treatment is administered influences on it.
Methods
Data from 530 patients with low or intermediate-1 risk MDS (according to FAB and WHO criteria) who received treatments with ESAs were recorded in SPRESAS. 458 of these patients had data available at diagnosis of MDS regarding comorbidities. These were classified as relevant (diabetes mellitus, atrial fibrillation, thromboembolic disease and renal impairement) and less relevant (arterial hypertension, others). Data from the size of the center where the treatment was administered were available in all the patients. Centers were distributed in levels according to the number of beds available: centers above 1000 beds were considered “level 1” centers, between 500 and 1000 as “level 2” and below 500 beds as “level 3”. Response was evaluated according IWG 2006 criteria.
Results
370 patients (80.8%) had at least one comorbidity, but when only relevant comorbidities were analyzed, only 252 patients (55.0%) presented at least one comorbidity (table 1). The presence of one comorbidity relevant or not did not show any impact on the overall response to ESAs (HR=1.441; p= 0.138 for one comorbidity; HR=1.278; p=0.223 for one relevant comorbidity). However, curiously, the presence of two or more comorbidities was associated with statistically significant better response to ESAs (68.9% response in patients with two or more comorbidities vs 64.2% in patients with less than two comorbidities; HR=3.014; p=0.004), the only difference between both groups was ferritine level while other variables with impact on response were similar in both groups (PB and BM blasts, Hb level, cytogenetics, transfusion dependency, IPSS and EPO level). Additionally, the presence of two or more relevant comorbidities was also associated with a better response (75.2% response in patients with two or more relevant comorbidities vs 64.0% in patients with less than two relevant comorbidities; HR= 4.126; p=0.001). These 2 groups were different regarding ferritine and EPO level. When the impact of the different comorbidities was studied, only hypertension showed influence on the overall response rate: patients with hypertension had statistically significant better response to ESAs (72.7%) than those who did not have hypertension (61.5%); p=0.015, both groups had similar characteristics regarding variables with impact on response. Regarding center size, 213 patients (40.2%) were treated in “level 1”; 174 (32.8%) in “level 2” and 143 (27.0%) in “level 3” centers. When the center level was analyzed we could verify that patients who were treated in the smallest “level 3” centers had a statistically significant higher overall response rate to ESAs (81.7%) when compared with “level 2” patients (66.0%) and “level 1” patients (52.8%), HR=3.995; p<0.001; there were less patients in transfusion dependence in “level 3” centers than in the others while other characteristics were similar in the 3 groups. No differences were observed regarding duration of ESAs response (p=0.098).
Conclusion
The present study shows that ESA treatment should not be avoided in patients with lower risk MDS with comorbidities, as response rates are similar to those of patients without. Patients treated in the smallest centers had the best response rates to ESA.

Session topic: E-poster
Keyword(s): Comorbidities, Myelodysplasia
{{ help_message }}
{{filter}}


