A PROGNOSTIC SCORING MODEL FOR PATIENTS TREATED WITH AZACITIDINE FOR MYELODYSPLASTIC SYNDROMES/ACUTE MYELOID LEUKEMIAS
(Abstract release date: 05/19/16)
EHA Library. Sato K. 06/09/16; 132769; E1220

Dr. Kazuya Sato
Contributions
Contributions
Abstract
Abstract: E1220
Type: Eposter Presentation
Background
Azacitidine (AZA) is widely used in clinical practice for patients with higher-risk myelodysplastic syndromes (MDS), as well as a proportion of patients with acute myeloid leukemia (AML). However, the predictive factors of a poor outcome in AZA-treated patients with MDS or AML have not been fully evaluated.
Aims
To clarify the risk factors (RFs) for patient survival, we retrospectively analyzed data of patients treated with AZA for MDS/AML.
Methods
We analyzed the clinical backgrounds, treatments, responses (hematological improvement: HI), survival, and prognostic factors of 31 patients with MDS/AML (MDS = 24, AML with myelodysplasia-related changes = 7) who were treated with AZA at our institute from 2011 to 2015. Expression of p53 protein in bone marrow cells (BMCs) was assessed immunohistochemically using a monoclonal mouse anti-human p53 protein antibody (Clone DO-7); p53 protein overexpression was defined as positivity in >50% of immunoreactive blasts. Overall survival (OS) was estimated from AZA treatment initiation via Kaplan–Meier analysis and compared with a log-rank test. RFs associated with OS were evaluated using a univariate (chi-squared test) or multivariate analysis (Cox proportional hazards model).
Results
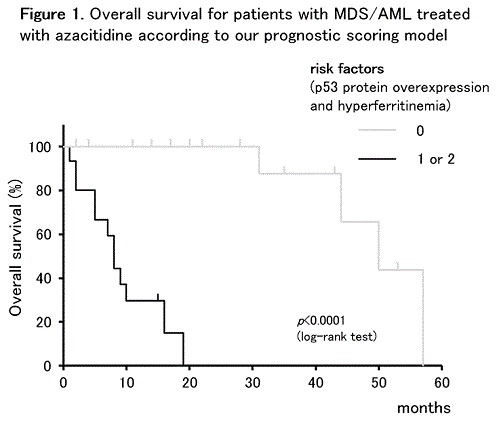
The patients included 24 men and 7 women with a median age of 73 (range: 44–89) years. One, 10, 9, and 11 patients belonged to the low, intermediate (int)-1, int-2, and high-risk International Prognostic Scoring System (IPSS) groups, respectively; 2, 11, 6, and 12 belonged to the low, intermediate, high (H) or very high (VH) revised IPSS (IPSS-R) groups, respectively. Complex karyotypes (CKs) and p53 protein overexpression were observed in BMCs from 9 and 7 patients, respectively. Patients received a median of 6 (range: 1–43) AZA cycles at a median interval of 35 (range: 28–56) days. Eighteen (58%) patients exhibited HI, and 14 (45%) died from disease progression. The estimated 4-year OS of all patients was 36.4%. RFs associated with OS in a univariate analysis were CKs (p = 0.0079), p53 protein overexpression in BMCs (p = 0.0309), high C-reactive protein level (p = 0.0018), serum ferritin >500 ng/ml (p = 0.0291), no HI (p = 0.0018), blood transfusion dependency (p = 0.0039), and IPSS-R H/VH risk group (p = 0.0484). Multivariate analysis identified p53 protein overexpression in BMCs (hazard ratio [HR] = 10.2, p = 0.0366) and serum ferritin >500 ng/ml (HR = 10.1, p = 0.0113) as independent RFs for OS. Two OS risk groups were defined according to scores from these risk factors: low risk (0) and high risk (1–2). The OS curves of AZA-treated patients with MDS/AML were significantly stratified into 2 risk groups using our scoring model (p <0.0001, Figure 1).
Conclusion
We demonstrated clear OS stratification of AZA-treated patients with MDS/AML into 2 risk groups according to our proposed scoring model using two risk factors, p53 protein overexpression and hyperferritinemia. Although further verification via prospective analysis is needed, these findings may provide valuable information for prognostic predictions of AZA-treated patients with MDS/AML.
Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Myelodysplasia, Survival prediction
Type: Eposter Presentation
Background
Azacitidine (AZA) is widely used in clinical practice for patients with higher-risk myelodysplastic syndromes (MDS), as well as a proportion of patients with acute myeloid leukemia (AML). However, the predictive factors of a poor outcome in AZA-treated patients with MDS or AML have not been fully evaluated.
Aims
To clarify the risk factors (RFs) for patient survival, we retrospectively analyzed data of patients treated with AZA for MDS/AML.
Methods
We analyzed the clinical backgrounds, treatments, responses (hematological improvement: HI), survival, and prognostic factors of 31 patients with MDS/AML (MDS = 24, AML with myelodysplasia-related changes = 7) who were treated with AZA at our institute from 2011 to 2015. Expression of p53 protein in bone marrow cells (BMCs) was assessed immunohistochemically using a monoclonal mouse anti-human p53 protein antibody (Clone DO-7); p53 protein overexpression was defined as positivity in >50% of immunoreactive blasts. Overall survival (OS) was estimated from AZA treatment initiation via Kaplan–Meier analysis and compared with a log-rank test. RFs associated with OS were evaluated using a univariate (chi-squared test) or multivariate analysis (Cox proportional hazards model).
Results
The patients included 24 men and 7 women with a median age of 73 (range: 44–89) years. One, 10, 9, and 11 patients belonged to the low, intermediate (int)-1, int-2, and high-risk International Prognostic Scoring System (IPSS) groups, respectively; 2, 11, 6, and 12 belonged to the low, intermediate, high (H) or very high (VH) revised IPSS (IPSS-R) groups, respectively. Complex karyotypes (CKs) and p53 protein overexpression were observed in BMCs from 9 and 7 patients, respectively. Patients received a median of 6 (range: 1–43) AZA cycles at a median interval of 35 (range: 28–56) days. Eighteen (58%) patients exhibited HI, and 14 (45%) died from disease progression. The estimated 4-year OS of all patients was 36.4%. RFs associated with OS in a univariate analysis were CKs (p = 0.0079), p53 protein overexpression in BMCs (p = 0.0309), high C-reactive protein level (p = 0.0018), serum ferritin >500 ng/ml (p = 0.0291), no HI (p = 0.0018), blood transfusion dependency (p = 0.0039), and IPSS-R H/VH risk group (p = 0.0484). Multivariate analysis identified p53 protein overexpression in BMCs (hazard ratio [HR] = 10.2, p = 0.0366) and serum ferritin >500 ng/ml (HR = 10.1, p = 0.0113) as independent RFs for OS. Two OS risk groups were defined according to scores from these risk factors: low risk (0) and high risk (1–2). The OS curves of AZA-treated patients with MDS/AML were significantly stratified into 2 risk groups using our scoring model (p <0.0001, Figure 1).
Conclusion
We demonstrated clear OS stratification of AZA-treated patients with MDS/AML into 2 risk groups according to our proposed scoring model using two risk factors, p53 protein overexpression and hyperferritinemia. Although further verification via prospective analysis is needed, these findings may provide valuable information for prognostic predictions of AZA-treated patients with MDS/AML.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Myelodysplasia, Survival prediction
Abstract: E1220
Type: Eposter Presentation
Background
Azacitidine (AZA) is widely used in clinical practice for patients with higher-risk myelodysplastic syndromes (MDS), as well as a proportion of patients with acute myeloid leukemia (AML). However, the predictive factors of a poor outcome in AZA-treated patients with MDS or AML have not been fully evaluated.
Aims
To clarify the risk factors (RFs) for patient survival, we retrospectively analyzed data of patients treated with AZA for MDS/AML.
Methods
We analyzed the clinical backgrounds, treatments, responses (hematological improvement: HI), survival, and prognostic factors of 31 patients with MDS/AML (MDS = 24, AML with myelodysplasia-related changes = 7) who were treated with AZA at our institute from 2011 to 2015. Expression of p53 protein in bone marrow cells (BMCs) was assessed immunohistochemically using a monoclonal mouse anti-human p53 protein antibody (Clone DO-7); p53 protein overexpression was defined as positivity in >50% of immunoreactive blasts. Overall survival (OS) was estimated from AZA treatment initiation via Kaplan–Meier analysis and compared with a log-rank test. RFs associated with OS were evaluated using a univariate (chi-squared test) or multivariate analysis (Cox proportional hazards model).
Results
The patients included 24 men and 7 women with a median age of 73 (range: 44–89) years. One, 10, 9, and 11 patients belonged to the low, intermediate (int)-1, int-2, and high-risk International Prognostic Scoring System (IPSS) groups, respectively; 2, 11, 6, and 12 belonged to the low, intermediate, high (H) or very high (VH) revised IPSS (IPSS-R) groups, respectively. Complex karyotypes (CKs) and p53 protein overexpression were observed in BMCs from 9 and 7 patients, respectively. Patients received a median of 6 (range: 1–43) AZA cycles at a median interval of 35 (range: 28–56) days. Eighteen (58%) patients exhibited HI, and 14 (45%) died from disease progression. The estimated 4-year OS of all patients was 36.4%. RFs associated with OS in a univariate analysis were CKs (p = 0.0079), p53 protein overexpression in BMCs (p = 0.0309), high C-reactive protein level (p = 0.0018), serum ferritin >500 ng/ml (p = 0.0291), no HI (p = 0.0018), blood transfusion dependency (p = 0.0039), and IPSS-R H/VH risk group (p = 0.0484). Multivariate analysis identified p53 protein overexpression in BMCs (hazard ratio [HR] = 10.2, p = 0.0366) and serum ferritin >500 ng/ml (HR = 10.1, p = 0.0113) as independent RFs for OS. Two OS risk groups were defined according to scores from these risk factors: low risk (0) and high risk (1–2). The OS curves of AZA-treated patients with MDS/AML were significantly stratified into 2 risk groups using our scoring model (p <0.0001, Figure 1).
Conclusion
We demonstrated clear OS stratification of AZA-treated patients with MDS/AML into 2 risk groups according to our proposed scoring model using two risk factors, p53 protein overexpression and hyperferritinemia. Although further verification via prospective analysis is needed, these findings may provide valuable information for prognostic predictions of AZA-treated patients with MDS/AML.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Myelodysplasia, Survival prediction
Type: Eposter Presentation
Background
Azacitidine (AZA) is widely used in clinical practice for patients with higher-risk myelodysplastic syndromes (MDS), as well as a proportion of patients with acute myeloid leukemia (AML). However, the predictive factors of a poor outcome in AZA-treated patients with MDS or AML have not been fully evaluated.
Aims
To clarify the risk factors (RFs) for patient survival, we retrospectively analyzed data of patients treated with AZA for MDS/AML.
Methods
We analyzed the clinical backgrounds, treatments, responses (hematological improvement: HI), survival, and prognostic factors of 31 patients with MDS/AML (MDS = 24, AML with myelodysplasia-related changes = 7) who were treated with AZA at our institute from 2011 to 2015. Expression of p53 protein in bone marrow cells (BMCs) was assessed immunohistochemically using a monoclonal mouse anti-human p53 protein antibody (Clone DO-7); p53 protein overexpression was defined as positivity in >50% of immunoreactive blasts. Overall survival (OS) was estimated from AZA treatment initiation via Kaplan–Meier analysis and compared with a log-rank test. RFs associated with OS were evaluated using a univariate (chi-squared test) or multivariate analysis (Cox proportional hazards model).
Results
The patients included 24 men and 7 women with a median age of 73 (range: 44–89) years. One, 10, 9, and 11 patients belonged to the low, intermediate (int)-1, int-2, and high-risk International Prognostic Scoring System (IPSS) groups, respectively; 2, 11, 6, and 12 belonged to the low, intermediate, high (H) or very high (VH) revised IPSS (IPSS-R) groups, respectively. Complex karyotypes (CKs) and p53 protein overexpression were observed in BMCs from 9 and 7 patients, respectively. Patients received a median of 6 (range: 1–43) AZA cycles at a median interval of 35 (range: 28–56) days. Eighteen (58%) patients exhibited HI, and 14 (45%) died from disease progression. The estimated 4-year OS of all patients was 36.4%. RFs associated with OS in a univariate analysis were CKs (p = 0.0079), p53 protein overexpression in BMCs (p = 0.0309), high C-reactive protein level (p = 0.0018), serum ferritin >500 ng/ml (p = 0.0291), no HI (p = 0.0018), blood transfusion dependency (p = 0.0039), and IPSS-R H/VH risk group (p = 0.0484). Multivariate analysis identified p53 protein overexpression in BMCs (hazard ratio [HR] = 10.2, p = 0.0366) and serum ferritin >500 ng/ml (HR = 10.1, p = 0.0113) as independent RFs for OS. Two OS risk groups were defined according to scores from these risk factors: low risk (0) and high risk (1–2). The OS curves of AZA-treated patients with MDS/AML were significantly stratified into 2 risk groups using our scoring model (p <0.0001, Figure 1).
Conclusion
We demonstrated clear OS stratification of AZA-treated patients with MDS/AML into 2 risk groups according to our proposed scoring model using two risk factors, p53 protein overexpression and hyperferritinemia. Although further verification via prospective analysis is needed, these findings may provide valuable information for prognostic predictions of AZA-treated patients with MDS/AML.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Myelodysplasia, Survival prediction
{{ help_message }}
{{filter}}


