CHARACTERIZATION OF TREATMENT PATTERNS AND OUTCOMES IN PATIENTS WITH MYELODYSPLASTIC SYNDROMES: ANALYSIS OF UNITED STATES COMMERCIAL CLAIMS DATABASE
(Abstract release date: 05/19/16)
EHA Library. Mehra M. 06/09/16; 132762; E1213
Disclosure(s): I am an employee of, and hold stock in, Janssen Research & Development.

Maneesha Mehra
Contributions
Contributions
Abstract
Abstract: E1213
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) constitute a heterogeneous form of blood cancer that primarily affects the elderly, and are characterized by anemia and other cytopenias as well as a high risk of transformation to acute myeloid leukemia (AML). Treatment, which is selected based on the International Prognostic Scoring System (IPSS), presence of cytopenia, and the presence of 5q deletion (del[5q] MDS), may include supportive care (eg, whole-blood and/or platelet transfusion), erythropoiesis-stimulating agents (ESA), hypomethylating agents, and lenalidomide for del(5q) MDS.
Aims
To characterize the burden of disease, treatment patterns, and outcomes of MDS patients using a large US database.
Methods
Optum’s integrated claims and Electronic Medical Record database (“Optum Database”) was retrospectively analyzed to identify adult patients with an index diagnosis of MDS between 2006 and 2014; to ensure index diagnosis, only patients with 365 days of retrospective data preceding the index diagnosis were included. Patients with baseline AML and patients younger than 18 years of age were excluded. MDS in patients was categorized as low-grade (ICD 9 code 238.72: Refractory anemia [RA], Refractory anemia with excess blasts-1 [RAEB-1], Refractory anemia with ringed sideroblasts [RARS], Refractory cytopenia with multilineage dysplasia [RCMD], Refractory cytopenia with multilineage dysplasia and ringed sideroblasts [RCMD-RS]), high-grade (ICD 9 code 238.73, which includes Refractory Anemia with Excess Blasts [RAEB] II), del(5q) (ICD 9 code: 238.74), or unspecified (ICD 9 code: 238.75) based on the earliest recorded ICD-9 diagnosis code based on French-American-British classification. Demographics and clinical outcomes were analyzed by descriptive summary. Treatment patterns were summarized by grouping treatments as “lenalidomide,” “azacitidine,” “decitabine,” “imatinib,” “chemotherapies,” “ESA,” and “others.” Kaplan-Meier survival analysis was performed to define progression to AML.
Results
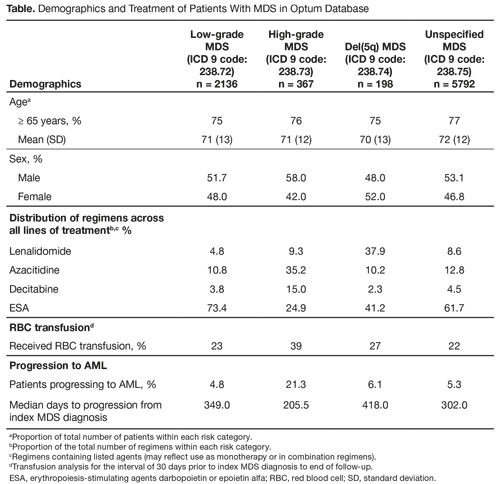
Of 10,465 MDS patients in the Optum Database, 8493 met the inclusion criteria and were evaluated over a median follow-up of 2.3 years; MDS was categorized as low-grade in 2136 (25.2%), high-grade in 367 (4.3%), del(5q) in 198 (2.3%), and unspecified in 5792 (68.2%) patients. Across all MDS categories, mean age at diagnosis was 70 to 72 years, 77% of patients were aged ≥ 65 years, roughly half were male, and the majority were white and non-Hispanic. In the overall cohort, 40% received ≥ 1 prescribed agent (46% in low-grade, 61% in high-grade, 42% in del[5q], and 36% in unspecified MDS). The most commonly used regimens were epoetin-alfa or darbepoetin in both low-grade and unspecified, hypomethylating agents (decitabine or azacitidine) in high-grade, and lenalidomide in del(5q) MDS (Table). In the overall cohort, 500 (5.9%) MDS patients progressed to AML, including 103 (4.8%) patients with low-grade and 78 (21.3%) with high-grade MDS. In the overall cohort, 38% of patients who received ≥ 1 regimen and 13% of patients without prescribed treatment went on to have a blood transfusion (Table).
Conclusion
Despite the variety of agents available, a significant proportion of MDS patients across all categories did not receive any treatment, and many received only supportive care. Rates of transformation to AML and transfusion dependence are consistent with published estimates.
Session topic: E-poster
Keyword(s): Myelodysplasia
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) constitute a heterogeneous form of blood cancer that primarily affects the elderly, and are characterized by anemia and other cytopenias as well as a high risk of transformation to acute myeloid leukemia (AML). Treatment, which is selected based on the International Prognostic Scoring System (IPSS), presence of cytopenia, and the presence of 5q deletion (del[5q] MDS), may include supportive care (eg, whole-blood and/or platelet transfusion), erythropoiesis-stimulating agents (ESA), hypomethylating agents, and lenalidomide for del(5q) MDS.
Aims
To characterize the burden of disease, treatment patterns, and outcomes of MDS patients using a large US database.
Methods
Optum’s integrated claims and Electronic Medical Record database (“Optum Database”) was retrospectively analyzed to identify adult patients with an index diagnosis of MDS between 2006 and 2014; to ensure index diagnosis, only patients with 365 days of retrospective data preceding the index diagnosis were included. Patients with baseline AML and patients younger than 18 years of age were excluded. MDS in patients was categorized as low-grade (ICD 9 code 238.72: Refractory anemia [RA], Refractory anemia with excess blasts-1 [RAEB-1], Refractory anemia with ringed sideroblasts [RARS], Refractory cytopenia with multilineage dysplasia [RCMD], Refractory cytopenia with multilineage dysplasia and ringed sideroblasts [RCMD-RS]), high-grade (ICD 9 code 238.73, which includes Refractory Anemia with Excess Blasts [RAEB] II), del(5q) (ICD 9 code: 238.74), or unspecified (ICD 9 code: 238.75) based on the earliest recorded ICD-9 diagnosis code based on French-American-British classification. Demographics and clinical outcomes were analyzed by descriptive summary. Treatment patterns were summarized by grouping treatments as “lenalidomide,” “azacitidine,” “decitabine,” “imatinib,” “chemotherapies,” “ESA,” and “others.” Kaplan-Meier survival analysis was performed to define progression to AML.
Results
Of 10,465 MDS patients in the Optum Database, 8493 met the inclusion criteria and were evaluated over a median follow-up of 2.3 years; MDS was categorized as low-grade in 2136 (25.2%), high-grade in 367 (4.3%), del(5q) in 198 (2.3%), and unspecified in 5792 (68.2%) patients. Across all MDS categories, mean age at diagnosis was 70 to 72 years, 77% of patients were aged ≥ 65 years, roughly half were male, and the majority were white and non-Hispanic. In the overall cohort, 40% received ≥ 1 prescribed agent (46% in low-grade, 61% in high-grade, 42% in del[5q], and 36% in unspecified MDS). The most commonly used regimens were epoetin-alfa or darbepoetin in both low-grade and unspecified, hypomethylating agents (decitabine or azacitidine) in high-grade, and lenalidomide in del(5q) MDS (Table). In the overall cohort, 500 (5.9%) MDS patients progressed to AML, including 103 (4.8%) patients with low-grade and 78 (21.3%) with high-grade MDS. In the overall cohort, 38% of patients who received ≥ 1 regimen and 13% of patients without prescribed treatment went on to have a blood transfusion (Table).
Conclusion
Despite the variety of agents available, a significant proportion of MDS patients across all categories did not receive any treatment, and many received only supportive care. Rates of transformation to AML and transfusion dependence are consistent with published estimates.

Session topic: E-poster
Keyword(s): Myelodysplasia
Abstract: E1213
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) constitute a heterogeneous form of blood cancer that primarily affects the elderly, and are characterized by anemia and other cytopenias as well as a high risk of transformation to acute myeloid leukemia (AML). Treatment, which is selected based on the International Prognostic Scoring System (IPSS), presence of cytopenia, and the presence of 5q deletion (del[5q] MDS), may include supportive care (eg, whole-blood and/or platelet transfusion), erythropoiesis-stimulating agents (ESA), hypomethylating agents, and lenalidomide for del(5q) MDS.
Aims
To characterize the burden of disease, treatment patterns, and outcomes of MDS patients using a large US database.
Methods
Optum’s integrated claims and Electronic Medical Record database (“Optum Database”) was retrospectively analyzed to identify adult patients with an index diagnosis of MDS between 2006 and 2014; to ensure index diagnosis, only patients with 365 days of retrospective data preceding the index diagnosis were included. Patients with baseline AML and patients younger than 18 years of age were excluded. MDS in patients was categorized as low-grade (ICD 9 code 238.72: Refractory anemia [RA], Refractory anemia with excess blasts-1 [RAEB-1], Refractory anemia with ringed sideroblasts [RARS], Refractory cytopenia with multilineage dysplasia [RCMD], Refractory cytopenia with multilineage dysplasia and ringed sideroblasts [RCMD-RS]), high-grade (ICD 9 code 238.73, which includes Refractory Anemia with Excess Blasts [RAEB] II), del(5q) (ICD 9 code: 238.74), or unspecified (ICD 9 code: 238.75) based on the earliest recorded ICD-9 diagnosis code based on French-American-British classification. Demographics and clinical outcomes were analyzed by descriptive summary. Treatment patterns were summarized by grouping treatments as “lenalidomide,” “azacitidine,” “decitabine,” “imatinib,” “chemotherapies,” “ESA,” and “others.” Kaplan-Meier survival analysis was performed to define progression to AML.
Results
Of 10,465 MDS patients in the Optum Database, 8493 met the inclusion criteria and were evaluated over a median follow-up of 2.3 years; MDS was categorized as low-grade in 2136 (25.2%), high-grade in 367 (4.3%), del(5q) in 198 (2.3%), and unspecified in 5792 (68.2%) patients. Across all MDS categories, mean age at diagnosis was 70 to 72 years, 77% of patients were aged ≥ 65 years, roughly half were male, and the majority were white and non-Hispanic. In the overall cohort, 40% received ≥ 1 prescribed agent (46% in low-grade, 61% in high-grade, 42% in del[5q], and 36% in unspecified MDS). The most commonly used regimens were epoetin-alfa or darbepoetin in both low-grade and unspecified, hypomethylating agents (decitabine or azacitidine) in high-grade, and lenalidomide in del(5q) MDS (Table). In the overall cohort, 500 (5.9%) MDS patients progressed to AML, including 103 (4.8%) patients with low-grade and 78 (21.3%) with high-grade MDS. In the overall cohort, 38% of patients who received ≥ 1 regimen and 13% of patients without prescribed treatment went on to have a blood transfusion (Table).
Conclusion
Despite the variety of agents available, a significant proportion of MDS patients across all categories did not receive any treatment, and many received only supportive care. Rates of transformation to AML and transfusion dependence are consistent with published estimates.

Session topic: E-poster
Keyword(s): Myelodysplasia
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) constitute a heterogeneous form of blood cancer that primarily affects the elderly, and are characterized by anemia and other cytopenias as well as a high risk of transformation to acute myeloid leukemia (AML). Treatment, which is selected based on the International Prognostic Scoring System (IPSS), presence of cytopenia, and the presence of 5q deletion (del[5q] MDS), may include supportive care (eg, whole-blood and/or platelet transfusion), erythropoiesis-stimulating agents (ESA), hypomethylating agents, and lenalidomide for del(5q) MDS.
Aims
To characterize the burden of disease, treatment patterns, and outcomes of MDS patients using a large US database.
Methods
Optum’s integrated claims and Electronic Medical Record database (“Optum Database”) was retrospectively analyzed to identify adult patients with an index diagnosis of MDS between 2006 and 2014; to ensure index diagnosis, only patients with 365 days of retrospective data preceding the index diagnosis were included. Patients with baseline AML and patients younger than 18 years of age were excluded. MDS in patients was categorized as low-grade (ICD 9 code 238.72: Refractory anemia [RA], Refractory anemia with excess blasts-1 [RAEB-1], Refractory anemia with ringed sideroblasts [RARS], Refractory cytopenia with multilineage dysplasia [RCMD], Refractory cytopenia with multilineage dysplasia and ringed sideroblasts [RCMD-RS]), high-grade (ICD 9 code 238.73, which includes Refractory Anemia with Excess Blasts [RAEB] II), del(5q) (ICD 9 code: 238.74), or unspecified (ICD 9 code: 238.75) based on the earliest recorded ICD-9 diagnosis code based on French-American-British classification. Demographics and clinical outcomes were analyzed by descriptive summary. Treatment patterns were summarized by grouping treatments as “lenalidomide,” “azacitidine,” “decitabine,” “imatinib,” “chemotherapies,” “ESA,” and “others.” Kaplan-Meier survival analysis was performed to define progression to AML.
Results
Of 10,465 MDS patients in the Optum Database, 8493 met the inclusion criteria and were evaluated over a median follow-up of 2.3 years; MDS was categorized as low-grade in 2136 (25.2%), high-grade in 367 (4.3%), del(5q) in 198 (2.3%), and unspecified in 5792 (68.2%) patients. Across all MDS categories, mean age at diagnosis was 70 to 72 years, 77% of patients were aged ≥ 65 years, roughly half were male, and the majority were white and non-Hispanic. In the overall cohort, 40% received ≥ 1 prescribed agent (46% in low-grade, 61% in high-grade, 42% in del[5q], and 36% in unspecified MDS). The most commonly used regimens were epoetin-alfa or darbepoetin in both low-grade and unspecified, hypomethylating agents (decitabine or azacitidine) in high-grade, and lenalidomide in del(5q) MDS (Table). In the overall cohort, 500 (5.9%) MDS patients progressed to AML, including 103 (4.8%) patients with low-grade and 78 (21.3%) with high-grade MDS. In the overall cohort, 38% of patients who received ≥ 1 regimen and 13% of patients without prescribed treatment went on to have a blood transfusion (Table).
Conclusion
Despite the variety of agents available, a significant proportion of MDS patients across all categories did not receive any treatment, and many received only supportive care. Rates of transformation to AML and transfusion dependence are consistent with published estimates.

Session topic: E-poster
Keyword(s): Myelodysplasia
{{ help_message }}
{{filter}}


