MOLECULAR ALTERATIONS ASSOCIATED WITH PROGRESSION OF MYELODYSPLASTIC SYNDROMES TO SECONDARY CHRONIC MYELOMONOCYTIC LEUKEMIA.
(Abstract release date: 05/19/16)
EHA Library. Sanna A. 06/09/16; 132759; E1210

Dr. Alessandro Sanna
Contributions
Contributions
Abstract
Abstract: E1210
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) are heterogeneous disorders characterized by ineffective hematopoiesis and risk of progression to acute myeloid leukemia. However, MDS may progress to clearly myeloproliferative neoplasms. Some reports have demonstrated that de novo MDS patients can transform to secondary chronic myelomonocytic leukemia (CMML) independently of the presence of relative monocytosis (>10%) at initial diagnosis of MDS (Rigolin et al, Haematologica 1997; Wang et al, Am J Clin Pathol 2006). Clinical parameters and cytogenetic alterations failed to correlate with probability to develop this type of progression.
Aims
The knowledge of the mechanisms at the basis of proliferative-non leukemic progression could help define the prognosis of this group of MDS patients. We postulated that specific acquired somatic mutations could be associated with the transformation from MDS to secondary CMML.
Methods
Seven IPSS lower risk MDS patients (pts) that progressed to CMML were included in the study. The mutational profile was characterized using a TruSeq Custom Amplicon panel (Illumina) targeting the 57 recurrently mutated genes in myeloid malignancies. Around 250 ng of genomic DNA extracted from mononuclear cells obtained at diagnosis (MDS phase) and at progression (CMML phase) were used to prepare sequencing libraries following the Illumina standard protocol. Samples were run on an Illumina MiSeq and variants were annotated by ANNOVAR. Detected variants were distilled on the basis of their exonic function, allele frequency, the presence in variants databases, and several prediction scores.
Results
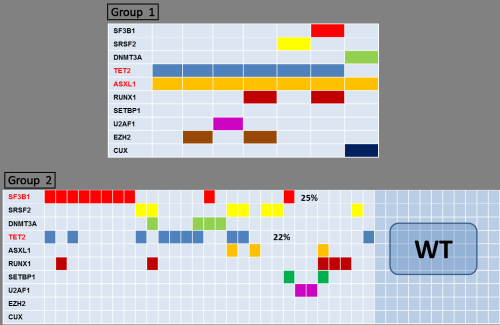
At diagnosis, 2 pts had RA and 6 pts had RCMD and at progression 5 pts presented a CMML-1 and 2 pts a CMML-2 (mean time to progression: 20 months). The total WBC count in the CMML phase was significantly higher than that of the MDS phase (mean, 24.4×109/L vs 4.04×109/L; P= 0.03). The mean hemoglobin level, platelet count, and blast count were not different between the MDS and CMML phases. In both phases, 3 pts presented a normal karyotype, 1 case a monosomy 7, 1 patient a trisomy 8 and 1 patient a trisomy 14. Only 1 patient presented a new trisomy 8 at progression. The most frequently mutated genes at MDS diagnosis were TET2 and ASXL1 (mutated in 7 and 6 pts, respectively). Only two pts presented new mutations in the progression CMML sample: one patient in the SETBP1 gene and the other one in the DNMT3A gene. The mutational profiling of these 7 MDS pts at diagnosis (group 1) was compared with that of a second cohort of 40 low-intermediate1 MDS pts (group 2) that did not progress (mean follow up: 25 months) (Figure 1). The number of mutations was higher in group 1 (100% pts presented > 3 mutations) than group 2 (80% of pts presented 0-2 mutations). The most commonly mutated genes differed between the two groups : ASXL1 (100%) and TET2 (86%) in group 1, while in group 2 were SF3B1 (25%) and TET2 (22%). In order to study the role of ASXL1 and TET2 mutations in the progression we compared variant allele frequency (VAF) data: VAF of ASXL1 was significantly higher in CMML than in MDS phase (38 vs 21; P=0.02), while VAF of TET2 did not shown differences.
Conclusion
This analysis of the rare type of progression from frank low risk MDS to MDS/MPN suggests that number of somatic mutations and presence of ASXL1 mutations during the MDS phase is an important factor correlated with/predictive of proliferative-non leukemic progression. Increase in VAF of ASXL1 seems also important in determining progression.
Session topic: E-poster
Keyword(s): Mutation analysis, Progression
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) are heterogeneous disorders characterized by ineffective hematopoiesis and risk of progression to acute myeloid leukemia. However, MDS may progress to clearly myeloproliferative neoplasms. Some reports have demonstrated that de novo MDS patients can transform to secondary chronic myelomonocytic leukemia (CMML) independently of the presence of relative monocytosis (>10%) at initial diagnosis of MDS (Rigolin et al, Haematologica 1997; Wang et al, Am J Clin Pathol 2006). Clinical parameters and cytogenetic alterations failed to correlate with probability to develop this type of progression.
Aims
The knowledge of the mechanisms at the basis of proliferative-non leukemic progression could help define the prognosis of this group of MDS patients. We postulated that specific acquired somatic mutations could be associated with the transformation from MDS to secondary CMML.
Methods
Seven IPSS lower risk MDS patients (pts) that progressed to CMML were included in the study. The mutational profile was characterized using a TruSeq Custom Amplicon panel (Illumina) targeting the 57 recurrently mutated genes in myeloid malignancies. Around 250 ng of genomic DNA extracted from mononuclear cells obtained at diagnosis (MDS phase) and at progression (CMML phase) were used to prepare sequencing libraries following the Illumina standard protocol. Samples were run on an Illumina MiSeq and variants were annotated by ANNOVAR. Detected variants were distilled on the basis of their exonic function, allele frequency, the presence in variants databases, and several prediction scores.
Results
At diagnosis, 2 pts had RA and 6 pts had RCMD and at progression 5 pts presented a CMML-1 and 2 pts a CMML-2 (mean time to progression: 20 months). The total WBC count in the CMML phase was significantly higher than that of the MDS phase (mean, 24.4×109/L vs 4.04×109/L; P= 0.03). The mean hemoglobin level, platelet count, and blast count were not different between the MDS and CMML phases. In both phases, 3 pts presented a normal karyotype, 1 case a monosomy 7, 1 patient a trisomy 8 and 1 patient a trisomy 14. Only 1 patient presented a new trisomy 8 at progression. The most frequently mutated genes at MDS diagnosis were TET2 and ASXL1 (mutated in 7 and 6 pts, respectively). Only two pts presented new mutations in the progression CMML sample: one patient in the SETBP1 gene and the other one in the DNMT3A gene. The mutational profiling of these 7 MDS pts at diagnosis (group 1) was compared with that of a second cohort of 40 low-intermediate1 MDS pts (group 2) that did not progress (mean follow up: 25 months) (Figure 1). The number of mutations was higher in group 1 (100% pts presented > 3 mutations) than group 2 (80% of pts presented 0-2 mutations). The most commonly mutated genes differed between the two groups : ASXL1 (100%) and TET2 (86%) in group 1, while in group 2 were SF3B1 (25%) and TET2 (22%). In order to study the role of ASXL1 and TET2 mutations in the progression we compared variant allele frequency (VAF) data: VAF of ASXL1 was significantly higher in CMML than in MDS phase (38 vs 21; P=0.02), while VAF of TET2 did not shown differences.
Conclusion
This analysis of the rare type of progression from frank low risk MDS to MDS/MPN suggests that number of somatic mutations and presence of ASXL1 mutations during the MDS phase is an important factor correlated with/predictive of proliferative-non leukemic progression. Increase in VAF of ASXL1 seems also important in determining progression.

Session topic: E-poster
Keyword(s): Mutation analysis, Progression
Abstract: E1210
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) are heterogeneous disorders characterized by ineffective hematopoiesis and risk of progression to acute myeloid leukemia. However, MDS may progress to clearly myeloproliferative neoplasms. Some reports have demonstrated that de novo MDS patients can transform to secondary chronic myelomonocytic leukemia (CMML) independently of the presence of relative monocytosis (>10%) at initial diagnosis of MDS (Rigolin et al, Haematologica 1997; Wang et al, Am J Clin Pathol 2006). Clinical parameters and cytogenetic alterations failed to correlate with probability to develop this type of progression.
Aims
The knowledge of the mechanisms at the basis of proliferative-non leukemic progression could help define the prognosis of this group of MDS patients. We postulated that specific acquired somatic mutations could be associated with the transformation from MDS to secondary CMML.
Methods
Seven IPSS lower risk MDS patients (pts) that progressed to CMML were included in the study. The mutational profile was characterized using a TruSeq Custom Amplicon panel (Illumina) targeting the 57 recurrently mutated genes in myeloid malignancies. Around 250 ng of genomic DNA extracted from mononuclear cells obtained at diagnosis (MDS phase) and at progression (CMML phase) were used to prepare sequencing libraries following the Illumina standard protocol. Samples were run on an Illumina MiSeq and variants were annotated by ANNOVAR. Detected variants were distilled on the basis of their exonic function, allele frequency, the presence in variants databases, and several prediction scores.
Results
At diagnosis, 2 pts had RA and 6 pts had RCMD and at progression 5 pts presented a CMML-1 and 2 pts a CMML-2 (mean time to progression: 20 months). The total WBC count in the CMML phase was significantly higher than that of the MDS phase (mean, 24.4×109/L vs 4.04×109/L; P= 0.03). The mean hemoglobin level, platelet count, and blast count were not different between the MDS and CMML phases. In both phases, 3 pts presented a normal karyotype, 1 case a monosomy 7, 1 patient a trisomy 8 and 1 patient a trisomy 14. Only 1 patient presented a new trisomy 8 at progression. The most frequently mutated genes at MDS diagnosis were TET2 and ASXL1 (mutated in 7 and 6 pts, respectively). Only two pts presented new mutations in the progression CMML sample: one patient in the SETBP1 gene and the other one in the DNMT3A gene. The mutational profiling of these 7 MDS pts at diagnosis (group 1) was compared with that of a second cohort of 40 low-intermediate1 MDS pts (group 2) that did not progress (mean follow up: 25 months) (Figure 1). The number of mutations was higher in group 1 (100% pts presented > 3 mutations) than group 2 (80% of pts presented 0-2 mutations). The most commonly mutated genes differed between the two groups : ASXL1 (100%) and TET2 (86%) in group 1, while in group 2 were SF3B1 (25%) and TET2 (22%). In order to study the role of ASXL1 and TET2 mutations in the progression we compared variant allele frequency (VAF) data: VAF of ASXL1 was significantly higher in CMML than in MDS phase (38 vs 21; P=0.02), while VAF of TET2 did not shown differences.
Conclusion
This analysis of the rare type of progression from frank low risk MDS to MDS/MPN suggests that number of somatic mutations and presence of ASXL1 mutations during the MDS phase is an important factor correlated with/predictive of proliferative-non leukemic progression. Increase in VAF of ASXL1 seems also important in determining progression.

Session topic: E-poster
Keyword(s): Mutation analysis, Progression
Type: Eposter Presentation
Background
Myelodysplastic syndromes (MDS) are heterogeneous disorders characterized by ineffective hematopoiesis and risk of progression to acute myeloid leukemia. However, MDS may progress to clearly myeloproliferative neoplasms. Some reports have demonstrated that de novo MDS patients can transform to secondary chronic myelomonocytic leukemia (CMML) independently of the presence of relative monocytosis (>10%) at initial diagnosis of MDS (Rigolin et al, Haematologica 1997; Wang et al, Am J Clin Pathol 2006). Clinical parameters and cytogenetic alterations failed to correlate with probability to develop this type of progression.
Aims
The knowledge of the mechanisms at the basis of proliferative-non leukemic progression could help define the prognosis of this group of MDS patients. We postulated that specific acquired somatic mutations could be associated with the transformation from MDS to secondary CMML.
Methods
Seven IPSS lower risk MDS patients (pts) that progressed to CMML were included in the study. The mutational profile was characterized using a TruSeq Custom Amplicon panel (Illumina) targeting the 57 recurrently mutated genes in myeloid malignancies. Around 250 ng of genomic DNA extracted from mononuclear cells obtained at diagnosis (MDS phase) and at progression (CMML phase) were used to prepare sequencing libraries following the Illumina standard protocol. Samples were run on an Illumina MiSeq and variants were annotated by ANNOVAR. Detected variants were distilled on the basis of their exonic function, allele frequency, the presence in variants databases, and several prediction scores.
Results
At diagnosis, 2 pts had RA and 6 pts had RCMD and at progression 5 pts presented a CMML-1 and 2 pts a CMML-2 (mean time to progression: 20 months). The total WBC count in the CMML phase was significantly higher than that of the MDS phase (mean, 24.4×109/L vs 4.04×109/L; P= 0.03). The mean hemoglobin level, platelet count, and blast count were not different between the MDS and CMML phases. In both phases, 3 pts presented a normal karyotype, 1 case a monosomy 7, 1 patient a trisomy 8 and 1 patient a trisomy 14. Only 1 patient presented a new trisomy 8 at progression. The most frequently mutated genes at MDS diagnosis were TET2 and ASXL1 (mutated in 7 and 6 pts, respectively). Only two pts presented new mutations in the progression CMML sample: one patient in the SETBP1 gene and the other one in the DNMT3A gene. The mutational profiling of these 7 MDS pts at diagnosis (group 1) was compared with that of a second cohort of 40 low-intermediate1 MDS pts (group 2) that did not progress (mean follow up: 25 months) (Figure 1). The number of mutations was higher in group 1 (100% pts presented > 3 mutations) than group 2 (80% of pts presented 0-2 mutations). The most commonly mutated genes differed between the two groups : ASXL1 (100%) and TET2 (86%) in group 1, while in group 2 were SF3B1 (25%) and TET2 (22%). In order to study the role of ASXL1 and TET2 mutations in the progression we compared variant allele frequency (VAF) data: VAF of ASXL1 was significantly higher in CMML than in MDS phase (38 vs 21; P=0.02), while VAF of TET2 did not shown differences.
Conclusion
This analysis of the rare type of progression from frank low risk MDS to MDS/MPN suggests that number of somatic mutations and presence of ASXL1 mutations during the MDS phase is an important factor correlated with/predictive of proliferative-non leukemic progression. Increase in VAF of ASXL1 seems also important in determining progression.

Session topic: E-poster
Keyword(s): Mutation analysis, Progression
{{ help_message }}
{{filter}}


