ABT-199 EFFECTIVELY INDUCES APOPTOSIS IN HIGH-RISK MDS PROGENITOR CELLS IRRESPECTIVE OF PROGNOSTICALLY ADVERSE MUTATIONS OF TP53, EZH2, RUNX1, AND ASXL1
(Abstract release date: 05/19/16)
EHA Library. Kauschinger J. 06/09/16; 132742; E1193

Ms. Johanna Kauschinger
Contributions
Contributions
Abstract
Abstract: E1193
Type: Eposter Presentation
Background
Myelodysplastic Syndromes (MDS) is a group of heterogeneous diseases with a broad spectrum of clinical outcomes. As we could recently show, the BCL-2 selective inhibitor ABT-199 effectively induces apoptosis in leukemic progenitors, as well as in blast cells of patients with high-risk MDS and secondary acute myeloid leukemia (sAML), while the healthy progenitor cell population was only marginally affected. As current therapeutic strategies such as Azacytidine have only a short response duration alternative regimes are urgently needed.Classification systems such as the International Prognostic Scoring System (IPSS) help to estimate the overall survival and the risk of developing a sAML. Disease-related molecular abnormalities are not incorporated in these scoring systems at the moment. However mutations of TP53, EZH2, RUNX1, and ASXL1 have been shown to identify patients with a shorter survival than predicted by the IPSS.
Aims
We were interested in analyzing whether ABT-199 could overcome resistance to apoptosis in high-risk MDS/sAML despite prognostically adverse mutations in TP53, EZH2, RUNX1, and ASXL1.
Methods
Purified bone marrow mononuclear cells (BMMNC) were treated with 1µM ABT-199 or a soluble control (DMSO) for 72h in vitro. Apoptosis was analyzed by flow cytometry after staining for 7-AAD, Annexin V, and CD34 as a progenitor marker. The long-term effect was investigated by colony formation assay. Mutational analysis was performed by Sanger sequencing at a certified laboratory (Munich Leukemia Laboratory). In total, seven healthy bone marrow samples and 38 MDS/sAML bone marrow samples (20 patients with proven wildtype alleles for TP53, EZH2, RUNX1, and ASXL1 as well as 18 patients with mutations in at least one of the indicated genes) were investigated.
Results
Prognostically bad molecular genetics including mutations of TP53, EZH2, RUNX1, and ASXL1 did not affect the response to ABT-199 treatment in MDS progenitor cells. No difference in the induction of apoptosis was seen between mutated and not mutated samples.As expected, the effect of ABT-199 correlated with MDS disease progression to elevated clinical risk groups. In addition, the long-term effect of BCL-2 inhibition could be analyzed in three patients of the high-risk MDS/sAML group with proven mutations by colony formation assays. Here we show that ABT-199 effectively killed colony-forming stem/progenitor cells from patient samples of the high-risk MDS/sAML subgroup.
Conclusion
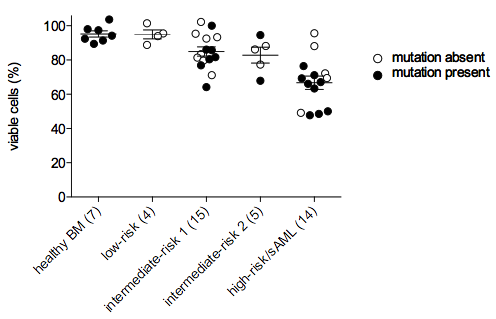
We conclude that ABT-199 is a promising drug for patients with high-risk MDS/sAML irrespective of prognostically adverse mutations of TP53, EZH2, RUNX1, and ASXL1.Figure legend: FICOLL-purified CD34+ BMMNC were treated for 72h with 1µM ABT-199 or a vehicle control (DMSO). Apoptosis was measured by flow cytometry. Each circle represents the ratio of viable cells after ABT-199 treatment to viable cells after sole vehicle treatment. Student’s t-test showed no significant difference between mutated and not mutated samples within one risk group. When compared with healthy controls, we found that induction of apoptosis was significantly increased in the stem/progenitor population from IPSS-classified patients with intermediate-risk 1 and 2 MDS, high-risk MDS, and sAML.
Session topic: E-poster
Keyword(s): Apoptosis, MDS
Type: Eposter Presentation
Background
Myelodysplastic Syndromes (MDS) is a group of heterogeneous diseases with a broad spectrum of clinical outcomes. As we could recently show, the BCL-2 selective inhibitor ABT-199 effectively induces apoptosis in leukemic progenitors, as well as in blast cells of patients with high-risk MDS and secondary acute myeloid leukemia (sAML), while the healthy progenitor cell population was only marginally affected. As current therapeutic strategies such as Azacytidine have only a short response duration alternative regimes are urgently needed.Classification systems such as the International Prognostic Scoring System (IPSS) help to estimate the overall survival and the risk of developing a sAML. Disease-related molecular abnormalities are not incorporated in these scoring systems at the moment. However mutations of TP53, EZH2, RUNX1, and ASXL1 have been shown to identify patients with a shorter survival than predicted by the IPSS.
Aims
We were interested in analyzing whether ABT-199 could overcome resistance to apoptosis in high-risk MDS/sAML despite prognostically adverse mutations in TP53, EZH2, RUNX1, and ASXL1.
Methods
Purified bone marrow mononuclear cells (BMMNC) were treated with 1µM ABT-199 or a soluble control (DMSO) for 72h in vitro. Apoptosis was analyzed by flow cytometry after staining for 7-AAD, Annexin V, and CD34 as a progenitor marker. The long-term effect was investigated by colony formation assay. Mutational analysis was performed by Sanger sequencing at a certified laboratory (Munich Leukemia Laboratory). In total, seven healthy bone marrow samples and 38 MDS/sAML bone marrow samples (20 patients with proven wildtype alleles for TP53, EZH2, RUNX1, and ASXL1 as well as 18 patients with mutations in at least one of the indicated genes) were investigated.
Results
Prognostically bad molecular genetics including mutations of TP53, EZH2, RUNX1, and ASXL1 did not affect the response to ABT-199 treatment in MDS progenitor cells. No difference in the induction of apoptosis was seen between mutated and not mutated samples.As expected, the effect of ABT-199 correlated with MDS disease progression to elevated clinical risk groups. In addition, the long-term effect of BCL-2 inhibition could be analyzed in three patients of the high-risk MDS/sAML group with proven mutations by colony formation assays. Here we show that ABT-199 effectively killed colony-forming stem/progenitor cells from patient samples of the high-risk MDS/sAML subgroup.
Conclusion
We conclude that ABT-199 is a promising drug for patients with high-risk MDS/sAML irrespective of prognostically adverse mutations of TP53, EZH2, RUNX1, and ASXL1.Figure legend: FICOLL-purified CD34+ BMMNC were treated for 72h with 1µM ABT-199 or a vehicle control (DMSO). Apoptosis was measured by flow cytometry. Each circle represents the ratio of viable cells after ABT-199 treatment to viable cells after sole vehicle treatment. Student’s t-test showed no significant difference between mutated and not mutated samples within one risk group. When compared with healthy controls, we found that induction of apoptosis was significantly increased in the stem/progenitor population from IPSS-classified patients with intermediate-risk 1 and 2 MDS, high-risk MDS, and sAML.

Session topic: E-poster
Keyword(s): Apoptosis, MDS
Abstract: E1193
Type: Eposter Presentation
Background
Myelodysplastic Syndromes (MDS) is a group of heterogeneous diseases with a broad spectrum of clinical outcomes. As we could recently show, the BCL-2 selective inhibitor ABT-199 effectively induces apoptosis in leukemic progenitors, as well as in blast cells of patients with high-risk MDS and secondary acute myeloid leukemia (sAML), while the healthy progenitor cell population was only marginally affected. As current therapeutic strategies such as Azacytidine have only a short response duration alternative regimes are urgently needed.Classification systems such as the International Prognostic Scoring System (IPSS) help to estimate the overall survival and the risk of developing a sAML. Disease-related molecular abnormalities are not incorporated in these scoring systems at the moment. However mutations of TP53, EZH2, RUNX1, and ASXL1 have been shown to identify patients with a shorter survival than predicted by the IPSS.
Aims
We were interested in analyzing whether ABT-199 could overcome resistance to apoptosis in high-risk MDS/sAML despite prognostically adverse mutations in TP53, EZH2, RUNX1, and ASXL1.
Methods
Purified bone marrow mononuclear cells (BMMNC) were treated with 1µM ABT-199 or a soluble control (DMSO) for 72h in vitro. Apoptosis was analyzed by flow cytometry after staining for 7-AAD, Annexin V, and CD34 as a progenitor marker. The long-term effect was investigated by colony formation assay. Mutational analysis was performed by Sanger sequencing at a certified laboratory (Munich Leukemia Laboratory). In total, seven healthy bone marrow samples and 38 MDS/sAML bone marrow samples (20 patients with proven wildtype alleles for TP53, EZH2, RUNX1, and ASXL1 as well as 18 patients with mutations in at least one of the indicated genes) were investigated.
Results
Prognostically bad molecular genetics including mutations of TP53, EZH2, RUNX1, and ASXL1 did not affect the response to ABT-199 treatment in MDS progenitor cells. No difference in the induction of apoptosis was seen between mutated and not mutated samples.As expected, the effect of ABT-199 correlated with MDS disease progression to elevated clinical risk groups. In addition, the long-term effect of BCL-2 inhibition could be analyzed in three patients of the high-risk MDS/sAML group with proven mutations by colony formation assays. Here we show that ABT-199 effectively killed colony-forming stem/progenitor cells from patient samples of the high-risk MDS/sAML subgroup.
Conclusion
We conclude that ABT-199 is a promising drug for patients with high-risk MDS/sAML irrespective of prognostically adverse mutations of TP53, EZH2, RUNX1, and ASXL1.Figure legend: FICOLL-purified CD34+ BMMNC were treated for 72h with 1µM ABT-199 or a vehicle control (DMSO). Apoptosis was measured by flow cytometry. Each circle represents the ratio of viable cells after ABT-199 treatment to viable cells after sole vehicle treatment. Student’s t-test showed no significant difference between mutated and not mutated samples within one risk group. When compared with healthy controls, we found that induction of apoptosis was significantly increased in the stem/progenitor population from IPSS-classified patients with intermediate-risk 1 and 2 MDS, high-risk MDS, and sAML.

Session topic: E-poster
Keyword(s): Apoptosis, MDS
Type: Eposter Presentation
Background
Myelodysplastic Syndromes (MDS) is a group of heterogeneous diseases with a broad spectrum of clinical outcomes. As we could recently show, the BCL-2 selective inhibitor ABT-199 effectively induces apoptosis in leukemic progenitors, as well as in blast cells of patients with high-risk MDS and secondary acute myeloid leukemia (sAML), while the healthy progenitor cell population was only marginally affected. As current therapeutic strategies such as Azacytidine have only a short response duration alternative regimes are urgently needed.Classification systems such as the International Prognostic Scoring System (IPSS) help to estimate the overall survival and the risk of developing a sAML. Disease-related molecular abnormalities are not incorporated in these scoring systems at the moment. However mutations of TP53, EZH2, RUNX1, and ASXL1 have been shown to identify patients with a shorter survival than predicted by the IPSS.
Aims
We were interested in analyzing whether ABT-199 could overcome resistance to apoptosis in high-risk MDS/sAML despite prognostically adverse mutations in TP53, EZH2, RUNX1, and ASXL1.
Methods
Purified bone marrow mononuclear cells (BMMNC) were treated with 1µM ABT-199 or a soluble control (DMSO) for 72h in vitro. Apoptosis was analyzed by flow cytometry after staining for 7-AAD, Annexin V, and CD34 as a progenitor marker. The long-term effect was investigated by colony formation assay. Mutational analysis was performed by Sanger sequencing at a certified laboratory (Munich Leukemia Laboratory). In total, seven healthy bone marrow samples and 38 MDS/sAML bone marrow samples (20 patients with proven wildtype alleles for TP53, EZH2, RUNX1, and ASXL1 as well as 18 patients with mutations in at least one of the indicated genes) were investigated.
Results
Prognostically bad molecular genetics including mutations of TP53, EZH2, RUNX1, and ASXL1 did not affect the response to ABT-199 treatment in MDS progenitor cells. No difference in the induction of apoptosis was seen between mutated and not mutated samples.As expected, the effect of ABT-199 correlated with MDS disease progression to elevated clinical risk groups. In addition, the long-term effect of BCL-2 inhibition could be analyzed in three patients of the high-risk MDS/sAML group with proven mutations by colony formation assays. Here we show that ABT-199 effectively killed colony-forming stem/progenitor cells from patient samples of the high-risk MDS/sAML subgroup.
Conclusion
We conclude that ABT-199 is a promising drug for patients with high-risk MDS/sAML irrespective of prognostically adverse mutations of TP53, EZH2, RUNX1, and ASXL1.Figure legend: FICOLL-purified CD34+ BMMNC were treated for 72h with 1µM ABT-199 or a vehicle control (DMSO). Apoptosis was measured by flow cytometry. Each circle represents the ratio of viable cells after ABT-199 treatment to viable cells after sole vehicle treatment. Student’s t-test showed no significant difference between mutated and not mutated samples within one risk group. When compared with healthy controls, we found that induction of apoptosis was significantly increased in the stem/progenitor population from IPSS-classified patients with intermediate-risk 1 and 2 MDS, high-risk MDS, and sAML.

Session topic: E-poster
Keyword(s): Apoptosis, MDS
{{ help_message }}
{{filter}}


