IMPACT OF STABLE DISEASE OR BETTER RESPONSES TO LENALIDOMIDE ON SURVIVAL OUTCOMES IN PATIENTS WITH RELAPSED/REFRACTORY MANTLE CELL LYMPHOMA: MCL-001 (EMERGE) AND MCL-002 (SPRINT) STUDIES
(Abstract release date: 05/19/16)
EHA Library. Goy A. 06/09/16; 132701; E1152

Dr. Andre Goy
Contributions
Contributions
Abstract
Abstract: E1152
Type: Eposter Presentation
Background
Patients with relapsed/refractory mantle cell lymphoma (R/R MCL) show poor overall survival (OS) after experiencing failure to immunochemotherapy and other treatment regimens, including bortezomib. Lenalidomide is an IMiD® immunomodulatory agent with direct and immune-mediated mechanisms of action that has demonstrated safety and efficacy (including meaningful disease stabilization rates) in multiple studies of R/R MCL.
Aims
Post hoc analysis of lenalidomide-treated patients from the MCL-001 and MCL-002 studies to determine the survival benefit based on response status (focusing on patients with stable disease [SD]) at landmark points in time.
Methods
Patients receiving oral lenalidomide 25 mg/day on days 1-21 of each 28-day cycle until disease progression or as tolerated were evaluated from 2 phase II studies: MCL-001 (post-bortezomib failure) and MCL-002 (randomized vs. investigator’s choice monotherapy). Patients provided informed consent for each study. Kaplan-Meier methods and unadjusted Cox regression models were used to support the landmark analyses at the start of cycles 3, 5, and 7. Survival data for patients with SD were compared with those with progressive disease (PD) or those responding with at least a partial response (PR) for each time point. Data cutoff for MCL-001 was March 20, 2013 and for MCL-002 was March 7, 2015.
Results
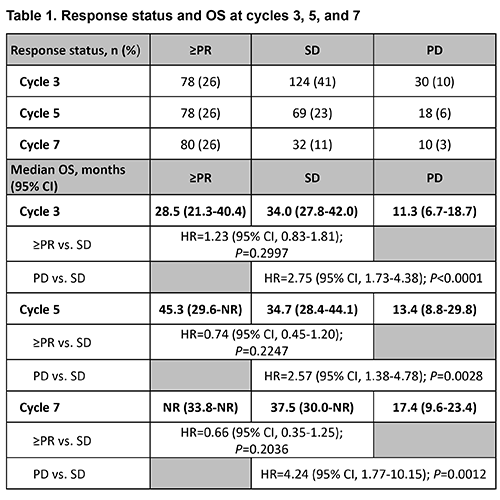
304 patients who received lenalidomide in MCL-001 (n=134) and MCL-002 (n=170) were included in the analysis. At baseline, patients had a median age of 68.0 years (66% were ≥65 years of age), 76% were male, 91% had stage III/IV MCL at diagnosis, and 52% had high tumor burden. Patients had received a median of 2 prior lines of antilymphoma therapy (range, 1-10); 48% were refractory (≤SD) to their last therapy. Baseline demographics and characteristics were similar for patients with ≥PR, SD, and PD. Response status at each cycle is shown in Table 1, with no response data for 24% of patients at cycle 3 due to early discontinuation, censoring or response being outside the time point window. Median OS in months by response group at cycle 3 was 28.5 for ≥PR, 34.0 for SD, and 11.3 for PD; at cycle 5: 45.3 for ≥PR, 34.7 for SD, and 13.4 for PD; and at cycle 7: not reached for ≥PR, 37.5 for SD, and 17.4 for PD. At all three time points, there was no significant difference in OS for patients with ≥PR vs. SD, whereas the median OS for patients with SD was significantly prolonged vs. PD. It is important to note that the SD and PD groups had a small number of patients at the later cycles.
Conclusion
Landmark analyses for lenalidomide-treated R/R MCL patients with SD at cycles 3, 5, and 7 had OS comparable to patients who achieved ≥PR. Overall, these data show that R/R MCL patients with either SD or ≥PR at these time points have significantly improved long-term outcomes compared to those with PD.
Session topic: E-poster
Keyword(s): Mantle cell lymphoma
Type: Eposter Presentation
Background
Patients with relapsed/refractory mantle cell lymphoma (R/R MCL) show poor overall survival (OS) after experiencing failure to immunochemotherapy and other treatment regimens, including bortezomib. Lenalidomide is an IMiD® immunomodulatory agent with direct and immune-mediated mechanisms of action that has demonstrated safety and efficacy (including meaningful disease stabilization rates) in multiple studies of R/R MCL.
Aims
Post hoc analysis of lenalidomide-treated patients from the MCL-001 and MCL-002 studies to determine the survival benefit based on response status (focusing on patients with stable disease [SD]) at landmark points in time.
Methods
Patients receiving oral lenalidomide 25 mg/day on days 1-21 of each 28-day cycle until disease progression or as tolerated were evaluated from 2 phase II studies: MCL-001 (post-bortezomib failure) and MCL-002 (randomized vs. investigator’s choice monotherapy). Patients provided informed consent for each study. Kaplan-Meier methods and unadjusted Cox regression models were used to support the landmark analyses at the start of cycles 3, 5, and 7. Survival data for patients with SD were compared with those with progressive disease (PD) or those responding with at least a partial response (PR) for each time point. Data cutoff for MCL-001 was March 20, 2013 and for MCL-002 was March 7, 2015.
Results
304 patients who received lenalidomide in MCL-001 (n=134) and MCL-002 (n=170) were included in the analysis. At baseline, patients had a median age of 68.0 years (66% were ≥65 years of age), 76% were male, 91% had stage III/IV MCL at diagnosis, and 52% had high tumor burden. Patients had received a median of 2 prior lines of antilymphoma therapy (range, 1-10); 48% were refractory (≤SD) to their last therapy. Baseline demographics and characteristics were similar for patients with ≥PR, SD, and PD. Response status at each cycle is shown in Table 1, with no response data for 24% of patients at cycle 3 due to early discontinuation, censoring or response being outside the time point window. Median OS in months by response group at cycle 3 was 28.5 for ≥PR, 34.0 for SD, and 11.3 for PD; at cycle 5: 45.3 for ≥PR, 34.7 for SD, and 13.4 for PD; and at cycle 7: not reached for ≥PR, 37.5 for SD, and 17.4 for PD. At all three time points, there was no significant difference in OS for patients with ≥PR vs. SD, whereas the median OS for patients with SD was significantly prolonged vs. PD. It is important to note that the SD and PD groups had a small number of patients at the later cycles.
Conclusion
Landmark analyses for lenalidomide-treated R/R MCL patients with SD at cycles 3, 5, and 7 had OS comparable to patients who achieved ≥PR. Overall, these data show that R/R MCL patients with either SD or ≥PR at these time points have significantly improved long-term outcomes compared to those with PD.

Session topic: E-poster
Keyword(s): Mantle cell lymphoma
Abstract: E1152
Type: Eposter Presentation
Background
Patients with relapsed/refractory mantle cell lymphoma (R/R MCL) show poor overall survival (OS) after experiencing failure to immunochemotherapy and other treatment regimens, including bortezomib. Lenalidomide is an IMiD® immunomodulatory agent with direct and immune-mediated mechanisms of action that has demonstrated safety and efficacy (including meaningful disease stabilization rates) in multiple studies of R/R MCL.
Aims
Post hoc analysis of lenalidomide-treated patients from the MCL-001 and MCL-002 studies to determine the survival benefit based on response status (focusing on patients with stable disease [SD]) at landmark points in time.
Methods
Patients receiving oral lenalidomide 25 mg/day on days 1-21 of each 28-day cycle until disease progression or as tolerated were evaluated from 2 phase II studies: MCL-001 (post-bortezomib failure) and MCL-002 (randomized vs. investigator’s choice monotherapy). Patients provided informed consent for each study. Kaplan-Meier methods and unadjusted Cox regression models were used to support the landmark analyses at the start of cycles 3, 5, and 7. Survival data for patients with SD were compared with those with progressive disease (PD) or those responding with at least a partial response (PR) for each time point. Data cutoff for MCL-001 was March 20, 2013 and for MCL-002 was March 7, 2015.
Results
304 patients who received lenalidomide in MCL-001 (n=134) and MCL-002 (n=170) were included in the analysis. At baseline, patients had a median age of 68.0 years (66% were ≥65 years of age), 76% were male, 91% had stage III/IV MCL at diagnosis, and 52% had high tumor burden. Patients had received a median of 2 prior lines of antilymphoma therapy (range, 1-10); 48% were refractory (≤SD) to their last therapy. Baseline demographics and characteristics were similar for patients with ≥PR, SD, and PD. Response status at each cycle is shown in Table 1, with no response data for 24% of patients at cycle 3 due to early discontinuation, censoring or response being outside the time point window. Median OS in months by response group at cycle 3 was 28.5 for ≥PR, 34.0 for SD, and 11.3 for PD; at cycle 5: 45.3 for ≥PR, 34.7 for SD, and 13.4 for PD; and at cycle 7: not reached for ≥PR, 37.5 for SD, and 17.4 for PD. At all three time points, there was no significant difference in OS for patients with ≥PR vs. SD, whereas the median OS for patients with SD was significantly prolonged vs. PD. It is important to note that the SD and PD groups had a small number of patients at the later cycles.
Conclusion
Landmark analyses for lenalidomide-treated R/R MCL patients with SD at cycles 3, 5, and 7 had OS comparable to patients who achieved ≥PR. Overall, these data show that R/R MCL patients with either SD or ≥PR at these time points have significantly improved long-term outcomes compared to those with PD.

Session topic: E-poster
Keyword(s): Mantle cell lymphoma
Type: Eposter Presentation
Background
Patients with relapsed/refractory mantle cell lymphoma (R/R MCL) show poor overall survival (OS) after experiencing failure to immunochemotherapy and other treatment regimens, including bortezomib. Lenalidomide is an IMiD® immunomodulatory agent with direct and immune-mediated mechanisms of action that has demonstrated safety and efficacy (including meaningful disease stabilization rates) in multiple studies of R/R MCL.
Aims
Post hoc analysis of lenalidomide-treated patients from the MCL-001 and MCL-002 studies to determine the survival benefit based on response status (focusing on patients with stable disease [SD]) at landmark points in time.
Methods
Patients receiving oral lenalidomide 25 mg/day on days 1-21 of each 28-day cycle until disease progression or as tolerated were evaluated from 2 phase II studies: MCL-001 (post-bortezomib failure) and MCL-002 (randomized vs. investigator’s choice monotherapy). Patients provided informed consent for each study. Kaplan-Meier methods and unadjusted Cox regression models were used to support the landmark analyses at the start of cycles 3, 5, and 7. Survival data for patients with SD were compared with those with progressive disease (PD) or those responding with at least a partial response (PR) for each time point. Data cutoff for MCL-001 was March 20, 2013 and for MCL-002 was March 7, 2015.
Results
304 patients who received lenalidomide in MCL-001 (n=134) and MCL-002 (n=170) were included in the analysis. At baseline, patients had a median age of 68.0 years (66% were ≥65 years of age), 76% were male, 91% had stage III/IV MCL at diagnosis, and 52% had high tumor burden. Patients had received a median of 2 prior lines of antilymphoma therapy (range, 1-10); 48% were refractory (≤SD) to their last therapy. Baseline demographics and characteristics were similar for patients with ≥PR, SD, and PD. Response status at each cycle is shown in Table 1, with no response data for 24% of patients at cycle 3 due to early discontinuation, censoring or response being outside the time point window. Median OS in months by response group at cycle 3 was 28.5 for ≥PR, 34.0 for SD, and 11.3 for PD; at cycle 5: 45.3 for ≥PR, 34.7 for SD, and 13.4 for PD; and at cycle 7: not reached for ≥PR, 37.5 for SD, and 17.4 for PD. At all three time points, there was no significant difference in OS for patients with ≥PR vs. SD, whereas the median OS for patients with SD was significantly prolonged vs. PD. It is important to note that the SD and PD groups had a small number of patients at the later cycles.
Conclusion
Landmark analyses for lenalidomide-treated R/R MCL patients with SD at cycles 3, 5, and 7 had OS comparable to patients who achieved ≥PR. Overall, these data show that R/R MCL patients with either SD or ≥PR at these time points have significantly improved long-term outcomes compared to those with PD.

Session topic: E-poster
Keyword(s): Mantle cell lymphoma
{{ help_message }}
{{filter}}


