BRENTUXIMAB VEDOTIN-BENDAMUSTINE COMBINATION FOR HODGKIN LYMPHOMA: EXPERIMENT WITH 8 PATIENTS.
(Abstract release date: 05/19/16)
EHA Library. Carola C. 06/09/16; 132695; E1146

Ms. Candice Carola
Contributions
Contributions
Abstract
Abstract: E1146
Type: Eposter Presentation
Background
Optimal treatment for patients (pts) with heavily pretreated Hodgkin lymphoma (HL) is controversial. Long term outcomes from autologous stem cell transplant (ASCT) in relapsed/refractory (R/R) HL are significantly better in pts achieving complete remission (CR) from salvage chemotherapy prior to ASCT. Brentuximab vedotin and bendamustine are highly active, as single agents, for pts with R/R HL and have manageable safety profile. Brentuximab is an antiCD30 monoclonal antibody conjugated to monomethyl auristatin E. Patients in CR after Brentuximab, particularly young pts and low risk early stage disease, did not necessarily benefit additional consolidative therapy (ASCT or allograft). Elsewhere, Brentuximab consolidation following ASCT should be proposed for pts with primary refractory disease, early relapse, or extra nodal disease.
Aims
To point brentuximab-bendamustine combination efficiency and safety for R/R HL pts.
Methods
We retrospectively analyzed 8 HL pts in 2 centers, treated with 90mg/m2 bendamustine on days 1 and 2, and 1.8mg/kg brentuximab on day 1, every 3 weeks. Six pts were R/R (including 1 post ASCT), 1 in partial response post chemotherapy and 1 in first line (contraindication to chemotherapy, heart and respiratory failures).
Results
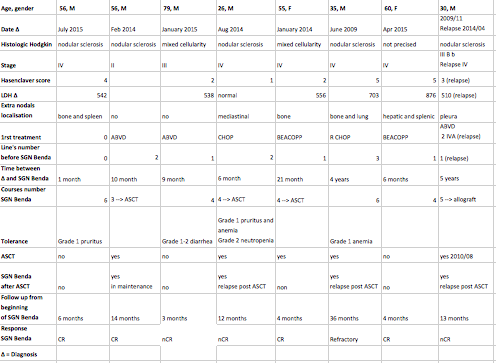
Median follow-up was 12 months [3-36] from beginning of treatment. Median chemotherapy treatment was 4 courses [2-6]. Four pts were in CR, 3 in nearly CR, 1 refractory. Three pts underwent subsequently ASCT (2 where in CR and 1 in nCR): 1 of them had brentuximab maintenance and 1 relapsed post ASCT. One other pt in nCR beneficiated from reduced intensity-conditioning allograft. All pts remained alive at follow-up. This combination was well tolerated, without grade 3-4 toxicities: 2 pts had grade 1 anemia, 1 of them grade 2 neutropenia.
Conclusion
This regimen represents a promising approach to optimize response rate prior to ASCT or allograft in pts with R/R HL. It could also be proposed to frail pts as frontline treatment.
Session topic: E-poster
Keyword(s): Autograft, Bendamustine, Hodgkin's lymphoma
Type: Eposter Presentation
Background
Optimal treatment for patients (pts) with heavily pretreated Hodgkin lymphoma (HL) is controversial. Long term outcomes from autologous stem cell transplant (ASCT) in relapsed/refractory (R/R) HL are significantly better in pts achieving complete remission (CR) from salvage chemotherapy prior to ASCT. Brentuximab vedotin and bendamustine are highly active, as single agents, for pts with R/R HL and have manageable safety profile. Brentuximab is an antiCD30 monoclonal antibody conjugated to monomethyl auristatin E. Patients in CR after Brentuximab, particularly young pts and low risk early stage disease, did not necessarily benefit additional consolidative therapy (ASCT or allograft). Elsewhere, Brentuximab consolidation following ASCT should be proposed for pts with primary refractory disease, early relapse, or extra nodal disease.
Aims
To point brentuximab-bendamustine combination efficiency and safety for R/R HL pts.
Methods
We retrospectively analyzed 8 HL pts in 2 centers, treated with 90mg/m2 bendamustine on days 1 and 2, and 1.8mg/kg brentuximab on day 1, every 3 weeks. Six pts were R/R (including 1 post ASCT), 1 in partial response post chemotherapy and 1 in first line (contraindication to chemotherapy, heart and respiratory failures).
Results
Median follow-up was 12 months [3-36] from beginning of treatment. Median chemotherapy treatment was 4 courses [2-6]. Four pts were in CR, 3 in nearly CR, 1 refractory. Three pts underwent subsequently ASCT (2 where in CR and 1 in nCR): 1 of them had brentuximab maintenance and 1 relapsed post ASCT. One other pt in nCR beneficiated from reduced intensity-conditioning allograft. All pts remained alive at follow-up. This combination was well tolerated, without grade 3-4 toxicities: 2 pts had grade 1 anemia, 1 of them grade 2 neutropenia.
Conclusion
This regimen represents a promising approach to optimize response rate prior to ASCT or allograft in pts with R/R HL. It could also be proposed to frail pts as frontline treatment.

Session topic: E-poster
Keyword(s): Autograft, Bendamustine, Hodgkin's lymphoma
Abstract: E1146
Type: Eposter Presentation
Background
Optimal treatment for patients (pts) with heavily pretreated Hodgkin lymphoma (HL) is controversial. Long term outcomes from autologous stem cell transplant (ASCT) in relapsed/refractory (R/R) HL are significantly better in pts achieving complete remission (CR) from salvage chemotherapy prior to ASCT. Brentuximab vedotin and bendamustine are highly active, as single agents, for pts with R/R HL and have manageable safety profile. Brentuximab is an antiCD30 monoclonal antibody conjugated to monomethyl auristatin E. Patients in CR after Brentuximab, particularly young pts and low risk early stage disease, did not necessarily benefit additional consolidative therapy (ASCT or allograft). Elsewhere, Brentuximab consolidation following ASCT should be proposed for pts with primary refractory disease, early relapse, or extra nodal disease.
Aims
To point brentuximab-bendamustine combination efficiency and safety for R/R HL pts.
Methods
We retrospectively analyzed 8 HL pts in 2 centers, treated with 90mg/m2 bendamustine on days 1 and 2, and 1.8mg/kg brentuximab on day 1, every 3 weeks. Six pts were R/R (including 1 post ASCT), 1 in partial response post chemotherapy and 1 in first line (contraindication to chemotherapy, heart and respiratory failures).
Results
Median follow-up was 12 months [3-36] from beginning of treatment. Median chemotherapy treatment was 4 courses [2-6]. Four pts were in CR, 3 in nearly CR, 1 refractory. Three pts underwent subsequently ASCT (2 where in CR and 1 in nCR): 1 of them had brentuximab maintenance and 1 relapsed post ASCT. One other pt in nCR beneficiated from reduced intensity-conditioning allograft. All pts remained alive at follow-up. This combination was well tolerated, without grade 3-4 toxicities: 2 pts had grade 1 anemia, 1 of them grade 2 neutropenia.
Conclusion
This regimen represents a promising approach to optimize response rate prior to ASCT or allograft in pts with R/R HL. It could also be proposed to frail pts as frontline treatment.

Session topic: E-poster
Keyword(s): Autograft, Bendamustine, Hodgkin's lymphoma
Type: Eposter Presentation
Background
Optimal treatment for patients (pts) with heavily pretreated Hodgkin lymphoma (HL) is controversial. Long term outcomes from autologous stem cell transplant (ASCT) in relapsed/refractory (R/R) HL are significantly better in pts achieving complete remission (CR) from salvage chemotherapy prior to ASCT. Brentuximab vedotin and bendamustine are highly active, as single agents, for pts with R/R HL and have manageable safety profile. Brentuximab is an antiCD30 monoclonal antibody conjugated to monomethyl auristatin E. Patients in CR after Brentuximab, particularly young pts and low risk early stage disease, did not necessarily benefit additional consolidative therapy (ASCT or allograft). Elsewhere, Brentuximab consolidation following ASCT should be proposed for pts with primary refractory disease, early relapse, or extra nodal disease.
Aims
To point brentuximab-bendamustine combination efficiency and safety for R/R HL pts.
Methods
We retrospectively analyzed 8 HL pts in 2 centers, treated with 90mg/m2 bendamustine on days 1 and 2, and 1.8mg/kg brentuximab on day 1, every 3 weeks. Six pts were R/R (including 1 post ASCT), 1 in partial response post chemotherapy and 1 in first line (contraindication to chemotherapy, heart and respiratory failures).
Results
Median follow-up was 12 months [3-36] from beginning of treatment. Median chemotherapy treatment was 4 courses [2-6]. Four pts were in CR, 3 in nearly CR, 1 refractory. Three pts underwent subsequently ASCT (2 where in CR and 1 in nCR): 1 of them had brentuximab maintenance and 1 relapsed post ASCT. One other pt in nCR beneficiated from reduced intensity-conditioning allograft. All pts remained alive at follow-up. This combination was well tolerated, without grade 3-4 toxicities: 2 pts had grade 1 anemia, 1 of them grade 2 neutropenia.
Conclusion
This regimen represents a promising approach to optimize response rate prior to ASCT or allograft in pts with R/R HL. It could also be proposed to frail pts as frontline treatment.

Session topic: E-poster
Keyword(s): Autograft, Bendamustine, Hodgkin's lymphoma
{{ help_message }}
{{filter}}


