ALTERATIONS OF BONE MARROW MICROENVIRONMENT IN PATIENTS WITH HEMATOLOGICAL DISORDERS AT THE DIAGNOSIS AND DURING THE TREATMENT
(Abstract release date: 05/19/16)
EHA Library. Sorokina T. 06/09/16; 132685; E1136

Dr. Tamara Sorokina
Contributions
Contributions
Abstract
Abstract: E1136
Type: Eposter Presentation
Background
Bone marrow (BM) microenvironment is involved in the initiation and propagation of normal hematopoiesis and hematological diseases. Leukemia and chemotherapy affect hematopoietic and stromal precursor cells. Multipotent mesenchymal stromal cells (MMSCs) are the essential element of hematopoietic microenvironment.
Aims
The study aimed to characterize MMSCs and their more differentiated progeny CFU-F derived from the BM of the patients with hematological disorders at diagnosis and during the treatment.
Methods
74 newly diagnosed cases (33 AML, 21 ALL, 20 CML) were involved in the study after informed consent. BM was aspirated prior to treatment (time-point 0) and at days 37, 100 and 180 since the beginning of treatment of acute leukemia and +3, +6 and +12 months for CML (time-points 1-3). MMSCs were cultured in aMEM with 10% fetal calf serum. Time to P0 and cumulative MMSC production after 3 passages were evaluated and CFU-F concentration was analyzed. The relative expression level (REL) of genes was measured by TaqMan RQ-PCR. As a control MMSCs and CFU-Fs from 88 healthy donors were used.
Results
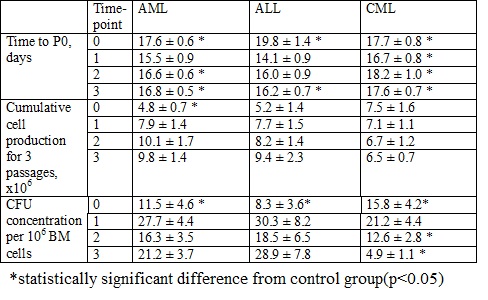
Time needed to reach P0 reflects the quantity of MMSC in the BM sample. The time to P0 in control group was 13.7±0.3 days. Its elongation in acute leukemia cultures at the time of the diagnosis (table) suggested the reduction of MMSC number probably due to leukemic expansion, while the longer time to P0 at time-points 2 and 3 could be explained by the therapy influence. In CML cultures time to P0 was significantly longer during the whole observation period due to the continuous therapy and maintaining disease.Cumulative MMSC production in control group was 7.1±1 x 106 cells. In patients with AML it was 1/3 of the donor’s at the disease manifestation with no difference at time-points 1-3, indicating the impaired proliferative abilities of MMSC at the AML manifestation. Cumulative MMSC production in patients with ALL and CML didn’t differ from donor’s. BM blast count did not correlate with MMSC production.CFU-F concentration in the BM of acute leukemia patients was significantly lower than donor’s (25.4±3.1 per 106 cells) at the time-point 0 with no difference at time-points 1-3. CFU-F concentration in the BM of CML patients was also nearly 40% lower than in control group at the time-point 0 with its following restoration at time-point 1 and subsequent drop (up to 5 fold lower) at time-point 3, reflecting the long-lasting lesion of CFU-Fs during the course of the disease.Gene expression analysis of MMSC from all patients revealed significant decrease in REL of VEGF and SOX9 genes at the disease manifestation that did not restore after the treatment. The REL of LIF was significantly increased at the disease manifestation, reflecting the efforts of MMSCs to inhibit leukemic proliferation. REL of PDGFRB and IL6 increased during the treatment. The treatment lead to the downregulation of FGF2, TGFB1 and 2. As FGF2 and TGFB inhibit the differentiation of MMSCs, their downregulation may refer to the effectiveness of therapy. In MMSCs from acute leukemia patients the ICAM and SPP1 genes were downregulated at all points, reflecting the mechanism of the blocking of MMSCs migration and differentiation during the stress conditions. The influence of chemotherapy lead to decrease in REL of SDF1, IL8, VCAM, IL1b1R1, JAG1, IGF1, FGFR1 and 2.
Conclusion
The study supports the major influence of leukemic cells and chemotherapy on the BM microenvironment. The two types of studied precursors are affected differently. Future studies are needed to evaluate the role of MMSCs in leukemia pathogenesis.
Session topic: E-poster
Keyword(s): Acute leukemia, Chronic myeloid leukemia, Mesenchymal stem cell, Microenvironment
Type: Eposter Presentation
Background
Bone marrow (BM) microenvironment is involved in the initiation and propagation of normal hematopoiesis and hematological diseases. Leukemia and chemotherapy affect hematopoietic and stromal precursor cells. Multipotent mesenchymal stromal cells (MMSCs) are the essential element of hematopoietic microenvironment.
Aims
The study aimed to characterize MMSCs and their more differentiated progeny CFU-F derived from the BM of the patients with hematological disorders at diagnosis and during the treatment.
Methods
74 newly diagnosed cases (33 AML, 21 ALL, 20 CML) were involved in the study after informed consent. BM was aspirated prior to treatment (time-point 0) and at days 37, 100 and 180 since the beginning of treatment of acute leukemia and +3, +6 and +12 months for CML (time-points 1-3). MMSCs were cultured in aMEM with 10% fetal calf serum. Time to P0 and cumulative MMSC production after 3 passages were evaluated and CFU-F concentration was analyzed. The relative expression level (REL) of genes was measured by TaqMan RQ-PCR. As a control MMSCs and CFU-Fs from 88 healthy donors were used.
Results
Time needed to reach P0 reflects the quantity of MMSC in the BM sample. The time to P0 in control group was 13.7±0.3 days. Its elongation in acute leukemia cultures at the time of the diagnosis (table) suggested the reduction of MMSC number probably due to leukemic expansion, while the longer time to P0 at time-points 2 and 3 could be explained by the therapy influence. In CML cultures time to P0 was significantly longer during the whole observation period due to the continuous therapy and maintaining disease.Cumulative MMSC production in control group was 7.1±1 x 106 cells. In patients with AML it was 1/3 of the donor’s at the disease manifestation with no difference at time-points 1-3, indicating the impaired proliferative abilities of MMSC at the AML manifestation. Cumulative MMSC production in patients with ALL and CML didn’t differ from donor’s. BM blast count did not correlate with MMSC production.CFU-F concentration in the BM of acute leukemia patients was significantly lower than donor’s (25.4±3.1 per 106 cells) at the time-point 0 with no difference at time-points 1-3. CFU-F concentration in the BM of CML patients was also nearly 40% lower than in control group at the time-point 0 with its following restoration at time-point 1 and subsequent drop (up to 5 fold lower) at time-point 3, reflecting the long-lasting lesion of CFU-Fs during the course of the disease.Gene expression analysis of MMSC from all patients revealed significant decrease in REL of VEGF and SOX9 genes at the disease manifestation that did not restore after the treatment. The REL of LIF was significantly increased at the disease manifestation, reflecting the efforts of MMSCs to inhibit leukemic proliferation. REL of PDGFRB and IL6 increased during the treatment. The treatment lead to the downregulation of FGF2, TGFB1 and 2. As FGF2 and TGFB inhibit the differentiation of MMSCs, their downregulation may refer to the effectiveness of therapy. In MMSCs from acute leukemia patients the ICAM and SPP1 genes were downregulated at all points, reflecting the mechanism of the blocking of MMSCs migration and differentiation during the stress conditions. The influence of chemotherapy lead to decrease in REL of SDF1, IL8, VCAM, IL1b1R1, JAG1, IGF1, FGFR1 and 2.
Conclusion
The study supports the major influence of leukemic cells and chemotherapy on the BM microenvironment. The two types of studied precursors are affected differently. Future studies are needed to evaluate the role of MMSCs in leukemia pathogenesis.

Session topic: E-poster
Keyword(s): Acute leukemia, Chronic myeloid leukemia, Mesenchymal stem cell, Microenvironment
Abstract: E1136
Type: Eposter Presentation
Background
Bone marrow (BM) microenvironment is involved in the initiation and propagation of normal hematopoiesis and hematological diseases. Leukemia and chemotherapy affect hematopoietic and stromal precursor cells. Multipotent mesenchymal stromal cells (MMSCs) are the essential element of hematopoietic microenvironment.
Aims
The study aimed to characterize MMSCs and their more differentiated progeny CFU-F derived from the BM of the patients with hematological disorders at diagnosis and during the treatment.
Methods
74 newly diagnosed cases (33 AML, 21 ALL, 20 CML) were involved in the study after informed consent. BM was aspirated prior to treatment (time-point 0) and at days 37, 100 and 180 since the beginning of treatment of acute leukemia and +3, +6 and +12 months for CML (time-points 1-3). MMSCs were cultured in aMEM with 10% fetal calf serum. Time to P0 and cumulative MMSC production after 3 passages were evaluated and CFU-F concentration was analyzed. The relative expression level (REL) of genes was measured by TaqMan RQ-PCR. As a control MMSCs and CFU-Fs from 88 healthy donors were used.
Results
Time needed to reach P0 reflects the quantity of MMSC in the BM sample. The time to P0 in control group was 13.7±0.3 days. Its elongation in acute leukemia cultures at the time of the diagnosis (table) suggested the reduction of MMSC number probably due to leukemic expansion, while the longer time to P0 at time-points 2 and 3 could be explained by the therapy influence. In CML cultures time to P0 was significantly longer during the whole observation period due to the continuous therapy and maintaining disease.Cumulative MMSC production in control group was 7.1±1 x 106 cells. In patients with AML it was 1/3 of the donor’s at the disease manifestation with no difference at time-points 1-3, indicating the impaired proliferative abilities of MMSC at the AML manifestation. Cumulative MMSC production in patients with ALL and CML didn’t differ from donor’s. BM blast count did not correlate with MMSC production.CFU-F concentration in the BM of acute leukemia patients was significantly lower than donor’s (25.4±3.1 per 106 cells) at the time-point 0 with no difference at time-points 1-3. CFU-F concentration in the BM of CML patients was also nearly 40% lower than in control group at the time-point 0 with its following restoration at time-point 1 and subsequent drop (up to 5 fold lower) at time-point 3, reflecting the long-lasting lesion of CFU-Fs during the course of the disease.Gene expression analysis of MMSC from all patients revealed significant decrease in REL of VEGF and SOX9 genes at the disease manifestation that did not restore after the treatment. The REL of LIF was significantly increased at the disease manifestation, reflecting the efforts of MMSCs to inhibit leukemic proliferation. REL of PDGFRB and IL6 increased during the treatment. The treatment lead to the downregulation of FGF2, TGFB1 and 2. As FGF2 and TGFB inhibit the differentiation of MMSCs, their downregulation may refer to the effectiveness of therapy. In MMSCs from acute leukemia patients the ICAM and SPP1 genes were downregulated at all points, reflecting the mechanism of the blocking of MMSCs migration and differentiation during the stress conditions. The influence of chemotherapy lead to decrease in REL of SDF1, IL8, VCAM, IL1b1R1, JAG1, IGF1, FGFR1 and 2.
Conclusion
The study supports the major influence of leukemic cells and chemotherapy on the BM microenvironment. The two types of studied precursors are affected differently. Future studies are needed to evaluate the role of MMSCs in leukemia pathogenesis.

Session topic: E-poster
Keyword(s): Acute leukemia, Chronic myeloid leukemia, Mesenchymal stem cell, Microenvironment
Type: Eposter Presentation
Background
Bone marrow (BM) microenvironment is involved in the initiation and propagation of normal hematopoiesis and hematological diseases. Leukemia and chemotherapy affect hematopoietic and stromal precursor cells. Multipotent mesenchymal stromal cells (MMSCs) are the essential element of hematopoietic microenvironment.
Aims
The study aimed to characterize MMSCs and their more differentiated progeny CFU-F derived from the BM of the patients with hematological disorders at diagnosis and during the treatment.
Methods
74 newly diagnosed cases (33 AML, 21 ALL, 20 CML) were involved in the study after informed consent. BM was aspirated prior to treatment (time-point 0) and at days 37, 100 and 180 since the beginning of treatment of acute leukemia and +3, +6 and +12 months for CML (time-points 1-3). MMSCs were cultured in aMEM with 10% fetal calf serum. Time to P0 and cumulative MMSC production after 3 passages were evaluated and CFU-F concentration was analyzed. The relative expression level (REL) of genes was measured by TaqMan RQ-PCR. As a control MMSCs and CFU-Fs from 88 healthy donors were used.
Results
Time needed to reach P0 reflects the quantity of MMSC in the BM sample. The time to P0 in control group was 13.7±0.3 days. Its elongation in acute leukemia cultures at the time of the diagnosis (table) suggested the reduction of MMSC number probably due to leukemic expansion, while the longer time to P0 at time-points 2 and 3 could be explained by the therapy influence. In CML cultures time to P0 was significantly longer during the whole observation period due to the continuous therapy and maintaining disease.Cumulative MMSC production in control group was 7.1±1 x 106 cells. In patients with AML it was 1/3 of the donor’s at the disease manifestation with no difference at time-points 1-3, indicating the impaired proliferative abilities of MMSC at the AML manifestation. Cumulative MMSC production in patients with ALL and CML didn’t differ from donor’s. BM blast count did not correlate with MMSC production.CFU-F concentration in the BM of acute leukemia patients was significantly lower than donor’s (25.4±3.1 per 106 cells) at the time-point 0 with no difference at time-points 1-3. CFU-F concentration in the BM of CML patients was also nearly 40% lower than in control group at the time-point 0 with its following restoration at time-point 1 and subsequent drop (up to 5 fold lower) at time-point 3, reflecting the long-lasting lesion of CFU-Fs during the course of the disease.Gene expression analysis of MMSC from all patients revealed significant decrease in REL of VEGF and SOX9 genes at the disease manifestation that did not restore after the treatment. The REL of LIF was significantly increased at the disease manifestation, reflecting the efforts of MMSCs to inhibit leukemic proliferation. REL of PDGFRB and IL6 increased during the treatment. The treatment lead to the downregulation of FGF2, TGFB1 and 2. As FGF2 and TGFB inhibit the differentiation of MMSCs, their downregulation may refer to the effectiveness of therapy. In MMSCs from acute leukemia patients the ICAM and SPP1 genes were downregulated at all points, reflecting the mechanism of the blocking of MMSCs migration and differentiation during the stress conditions. The influence of chemotherapy lead to decrease in REL of SDF1, IL8, VCAM, IL1b1R1, JAG1, IGF1, FGFR1 and 2.
Conclusion
The study supports the major influence of leukemic cells and chemotherapy on the BM microenvironment. The two types of studied precursors are affected differently. Future studies are needed to evaluate the role of MMSCs in leukemia pathogenesis.

Session topic: E-poster
Keyword(s): Acute leukemia, Chronic myeloid leukemia, Mesenchymal stem cell, Microenvironment
{{ help_message }}
{{filter}}


