SYNERGISTIC KILLING EFFECT OF A NOVEL TRIPLE-REGULATED ONCOLYTIC ADENOVIRUS CARRYING PROGRAMMED CELL DEATH 5 GENE AND DAUNORUBICIN ON HUMAN LEUKEMIC CELLS
(Abstract release date: 05/19/16)
EHA Library. Ruan G. 06/09/16; 132668; E1119

Prof. Guo-Rui Ruan
Contributions
Contributions
Abstract
Abstract: E1119
Type: Eposter Presentation
Background
The expression levels of programmed cell death 5 (PDCD5) are down-regulated in many malignancies. A novel triple-regulated conditionally replicating adenoviruses (CRAd) carrying PDCD5 gene expression cassette, SG611-PDCD5, alone could inhibit tumor growth in vitro and in vivo and protect selectively the normal cells.
Aims
The purpose of this study was to investigate the synergistic killing effect of SG611-PDCD5 and low-dose daunorubicin (DNR) on human leukemic cells in vitro and in vivo.
Methods
The antitumor efficacy was characterized in several leukemic cell lines in vitro and in xenograft models of human leukemic cell line in nude mice. A panel of leukemic cells was treated with different concentrations of DNR alone or in combination with different multiplicities of infection (MOI) of SG611-PDCD5. The cell viability was determined by using CCK-8 assay. Apoptosis was detected in whole living cells using flow cytometry or in paraffin-embedded tumor tissues using the TUNEL kit.
Results
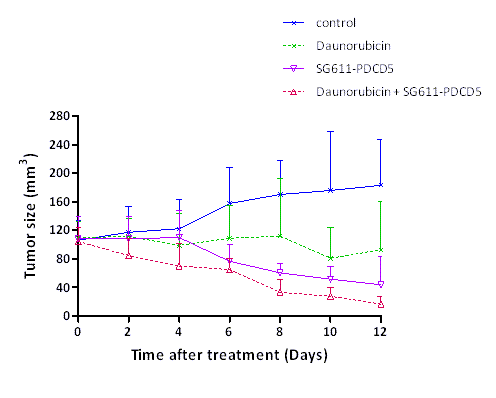
Combined SG611-PDCD5 and low-dose DNR showed synergetic effects in inhibiting the proliferation of human leukemic cell lines K562, MEG-01, KG-1a and TF-1. Synergistic effects in inducing apoptosis were observed both in K562 cells in vitro and in tumor tissues from xenograft models of K562 cells in nude mice. In K562 cells, the apoptotic percentages of SG611-PDCD5 at an MOI of 240 pfu/cell in combination with 0.12 ug/ml DNR were 72.5%, significant higher than that of SG611-PDCD5 alone (49.4%) or DNR alone (16.7%). TUNEL assay showed significantly more apoptotic cells in the SG611-PDCD5 plus DNR group than in SG611-PDCD5 group or in DNR group (25.0, 12.5 and 7.8 apoptotic cells/field, respectively, P<0.05). As shown in vivo experiment (Figure1), the tumor sizes were significantly decreased in combined treatment group on days 8 and 10 after treatment than those in DNR alone and SG611-PDCD5 alone (all P<0.05). Tumor sizes in the control, DNR, SG611-PDCD5 and DNR plus SG611-PDCD5 groups were 175.9 ± 82.5, 80.9 ± 42.8, 51.6 ± 17.4, and 27.6 ± 11.9 mm3 on day 10, respectively.
Conclusion
Combined treatment of SG611-PDCD5 and DNR achieves synergistic effects in inhibiting human leukemic cell growth in vitro and in vivo. This study may lead to development of new strategies for effective leukemia treatment with a potential reduction in systemic toxicity.
Session topic: E-poster
Keyword(s): Apoptosis, Gene therapy, Leukemia
Type: Eposter Presentation
Background
The expression levels of programmed cell death 5 (PDCD5) are down-regulated in many malignancies. A novel triple-regulated conditionally replicating adenoviruses (CRAd) carrying PDCD5 gene expression cassette, SG611-PDCD5, alone could inhibit tumor growth in vitro and in vivo and protect selectively the normal cells.
Aims
The purpose of this study was to investigate the synergistic killing effect of SG611-PDCD5 and low-dose daunorubicin (DNR) on human leukemic cells in vitro and in vivo.
Methods
The antitumor efficacy was characterized in several leukemic cell lines in vitro and in xenograft models of human leukemic cell line in nude mice. A panel of leukemic cells was treated with different concentrations of DNR alone or in combination with different multiplicities of infection (MOI) of SG611-PDCD5. The cell viability was determined by using CCK-8 assay. Apoptosis was detected in whole living cells using flow cytometry or in paraffin-embedded tumor tissues using the TUNEL kit.
Results
Combined SG611-PDCD5 and low-dose DNR showed synergetic effects in inhibiting the proliferation of human leukemic cell lines K562, MEG-01, KG-1a and TF-1. Synergistic effects in inducing apoptosis were observed both in K562 cells in vitro and in tumor tissues from xenograft models of K562 cells in nude mice. In K562 cells, the apoptotic percentages of SG611-PDCD5 at an MOI of 240 pfu/cell in combination with 0.12 ug/ml DNR were 72.5%, significant higher than that of SG611-PDCD5 alone (49.4%) or DNR alone (16.7%). TUNEL assay showed significantly more apoptotic cells in the SG611-PDCD5 plus DNR group than in SG611-PDCD5 group or in DNR group (25.0, 12.5 and 7.8 apoptotic cells/field, respectively, P<0.05). As shown in vivo experiment (Figure1), the tumor sizes were significantly decreased in combined treatment group on days 8 and 10 after treatment than those in DNR alone and SG611-PDCD5 alone (all P<0.05). Tumor sizes in the control, DNR, SG611-PDCD5 and DNR plus SG611-PDCD5 groups were 175.9 ± 82.5, 80.9 ± 42.8, 51.6 ± 17.4, and 27.6 ± 11.9 mm3 on day 10, respectively.
Conclusion
Combined treatment of SG611-PDCD5 and DNR achieves synergistic effects in inhibiting human leukemic cell growth in vitro and in vivo. This study may lead to development of new strategies for effective leukemia treatment with a potential reduction in systemic toxicity.

Session topic: E-poster
Keyword(s): Apoptosis, Gene therapy, Leukemia
Abstract: E1119
Type: Eposter Presentation
Background
The expression levels of programmed cell death 5 (PDCD5) are down-regulated in many malignancies. A novel triple-regulated conditionally replicating adenoviruses (CRAd) carrying PDCD5 gene expression cassette, SG611-PDCD5, alone could inhibit tumor growth in vitro and in vivo and protect selectively the normal cells.
Aims
The purpose of this study was to investigate the synergistic killing effect of SG611-PDCD5 and low-dose daunorubicin (DNR) on human leukemic cells in vitro and in vivo.
Methods
The antitumor efficacy was characterized in several leukemic cell lines in vitro and in xenograft models of human leukemic cell line in nude mice. A panel of leukemic cells was treated with different concentrations of DNR alone or in combination with different multiplicities of infection (MOI) of SG611-PDCD5. The cell viability was determined by using CCK-8 assay. Apoptosis was detected in whole living cells using flow cytometry or in paraffin-embedded tumor tissues using the TUNEL kit.
Results
Combined SG611-PDCD5 and low-dose DNR showed synergetic effects in inhibiting the proliferation of human leukemic cell lines K562, MEG-01, KG-1a and TF-1. Synergistic effects in inducing apoptosis were observed both in K562 cells in vitro and in tumor tissues from xenograft models of K562 cells in nude mice. In K562 cells, the apoptotic percentages of SG611-PDCD5 at an MOI of 240 pfu/cell in combination with 0.12 ug/ml DNR were 72.5%, significant higher than that of SG611-PDCD5 alone (49.4%) or DNR alone (16.7%). TUNEL assay showed significantly more apoptotic cells in the SG611-PDCD5 plus DNR group than in SG611-PDCD5 group or in DNR group (25.0, 12.5 and 7.8 apoptotic cells/field, respectively, P<0.05). As shown in vivo experiment (Figure1), the tumor sizes were significantly decreased in combined treatment group on days 8 and 10 after treatment than those in DNR alone and SG611-PDCD5 alone (all P<0.05). Tumor sizes in the control, DNR, SG611-PDCD5 and DNR plus SG611-PDCD5 groups were 175.9 ± 82.5, 80.9 ± 42.8, 51.6 ± 17.4, and 27.6 ± 11.9 mm3 on day 10, respectively.
Conclusion
Combined treatment of SG611-PDCD5 and DNR achieves synergistic effects in inhibiting human leukemic cell growth in vitro and in vivo. This study may lead to development of new strategies for effective leukemia treatment with a potential reduction in systemic toxicity.

Session topic: E-poster
Keyword(s): Apoptosis, Gene therapy, Leukemia
Type: Eposter Presentation
Background
The expression levels of programmed cell death 5 (PDCD5) are down-regulated in many malignancies. A novel triple-regulated conditionally replicating adenoviruses (CRAd) carrying PDCD5 gene expression cassette, SG611-PDCD5, alone could inhibit tumor growth in vitro and in vivo and protect selectively the normal cells.
Aims
The purpose of this study was to investigate the synergistic killing effect of SG611-PDCD5 and low-dose daunorubicin (DNR) on human leukemic cells in vitro and in vivo.
Methods
The antitumor efficacy was characterized in several leukemic cell lines in vitro and in xenograft models of human leukemic cell line in nude mice. A panel of leukemic cells was treated with different concentrations of DNR alone or in combination with different multiplicities of infection (MOI) of SG611-PDCD5. The cell viability was determined by using CCK-8 assay. Apoptosis was detected in whole living cells using flow cytometry or in paraffin-embedded tumor tissues using the TUNEL kit.
Results
Combined SG611-PDCD5 and low-dose DNR showed synergetic effects in inhibiting the proliferation of human leukemic cell lines K562, MEG-01, KG-1a and TF-1. Synergistic effects in inducing apoptosis were observed both in K562 cells in vitro and in tumor tissues from xenograft models of K562 cells in nude mice. In K562 cells, the apoptotic percentages of SG611-PDCD5 at an MOI of 240 pfu/cell in combination with 0.12 ug/ml DNR were 72.5%, significant higher than that of SG611-PDCD5 alone (49.4%) or DNR alone (16.7%). TUNEL assay showed significantly more apoptotic cells in the SG611-PDCD5 plus DNR group than in SG611-PDCD5 group or in DNR group (25.0, 12.5 and 7.8 apoptotic cells/field, respectively, P<0.05). As shown in vivo experiment (Figure1), the tumor sizes were significantly decreased in combined treatment group on days 8 and 10 after treatment than those in DNR alone and SG611-PDCD5 alone (all P<0.05). Tumor sizes in the control, DNR, SG611-PDCD5 and DNR plus SG611-PDCD5 groups were 175.9 ± 82.5, 80.9 ± 42.8, 51.6 ± 17.4, and 27.6 ± 11.9 mm3 on day 10, respectively.
Conclusion
Combined treatment of SG611-PDCD5 and DNR achieves synergistic effects in inhibiting human leukemic cell growth in vitro and in vivo. This study may lead to development of new strategies for effective leukemia treatment with a potential reduction in systemic toxicity.

Session topic: E-poster
Keyword(s): Apoptosis, Gene therapy, Leukemia
{{ help_message }}
{{filter}}


