CONCURRENT OPTIMAL MOLECULAR AND CYTOGENETIC RESPONSES AT BOTH 3 AND 6 MONTHS PREDICT A HIGHER PROBABILITY OF MR4.5 ACHIEVEMENT IN PATIENTS WITH CHRONIC MYELOID LEUKEMIA TREATED WITH IMATINIB
(Abstract release date: 05/19/16)
EHA Library. Qin Y. 06/09/16; 132664; E1115

Dr. Ya-Zhen Qin
Contributions
Contributions
Abstract
Abstract: E1115
Type: Eposter Presentation
Background
Tyrosine kinase inhibitors (TKIs) have become the first line treatment of chronic myeloid leukemia (CML). Because early molecular and cytogenetic responses have been demonstrated to strongly predict long-term outcomes, the 2013 European LeukemiaNet (ELN) recommends that the patient early response should be defined by both molecular and cytogenetic tests to optimally direct the selection of subsequent treatments. The rapid achievement of a deep molecular response has become the treatment goal, therefore, the impact of the combination of early responses on it needs to be investigated.
Aims
We tried to evaluate the impact of the combined molecular and cytogenetic responses at 3 and 6 months on the achievement of molecular response of 4.5 (MR4.5) in imatinib treated CML patients.
Methods
A total of 228 newly diagnosed chronic phase CML patients treated with imatinib were included. At 3 and 6 months, they all were detected BCR-ABL transcript levels by real-time quantitative polymerase chain reaction (RQ-PCR) using peripheral blood samples, and performed karyotyping by standard G-banding techniques. Patients were grouped into 3-month and 6-month molecular optimal cytogenetic optimal (MOCO), molecular optimal cytogenetic warning (MOCW), molecular warning cytogenetic optimal (MWCO), molecular warning cytogenetic warning (MWCW) and failure groups according to the 2013 ELN recommendation. BCR-ABLIS (BCR-ABL levels according to the international scale) was serial followed up for molecular response evaluation.
Results
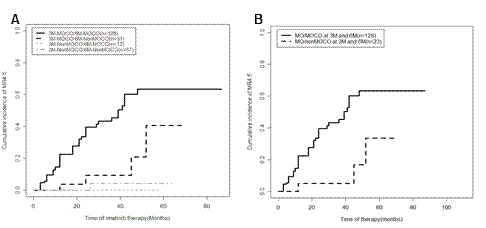
The median follow-up time was 27 months (range 8-94). For 3 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (8.3% [95% confidence interval (CI), 0-70.5%], 0, 0 and 0, all P>0.05), and they all were significantly lower than that of MOCO patients (35.5% [95% CI, 23.2-48.0%]; P=0.0047, 0.0057, 0.0089 and 0.0081, respectively). Similarly, for 6 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (5.6% [95% CI, 0-66.6%], 20.0% [95% CI, 0.2-67.0%], 0 and 0, all P>0.05), and they were all significantly lower than that of MOCO patients (41.6% [95% CI, 29.2-53.5%]; P=0.025, 0.021, 0.0005 and 0.0010, respectively). Next, responses at 3 and 6 months were combined. Three-month MOCO/6-month non-MOCO, 3-month non-MOCO/6-month MOCO and 3-month non-MOCO/6-month non-MOCO patients had similar 3-year MR4.5 rates (9.4% [95% CI, 0-56.5%], 0 and 4.2% [95% CI, 0-64.0%], P>0.05), and they were all significantly lower than that of 3-month MOCO/6-month MOCO patients (45.4% [95%CI, 32.9-57.1%]; P=0.0024, 0.0073 and <0.0001). This patient group also had significantly higher 3-year event-free survival (EFS) and progression-free survival rates (PFS) than those of patients with non-MOCO at 3 and/or 6 months (P<0.0001 and 0.0065) and had a significantly higher 3-year MR4.5 rate than that of patients with a 3-month/6-month molecularly optimal response but not a cytogenetically optimal response at 3 and/or 6 months (including 3-month MOCO/6-month MOCW, 3-month MOCW/6-month MOCO and 3-month MOCW/6-month MOCW, 5.0% [95%CI, 0-65.6%], P=0.0028).
Conclusion
The combination of early molecular and cytogenetic responses better predicts a deep molecular response in CML patients treated with imatinib. Concurrent optimal molecular and cytogenetic responses at both 3 and 6 months are associated with MR4.5 achievement.Grant support The Nature Science Foundation of China (81370637 and 81570130) and the Beijing Municipal Science and Technology Program (Z131100004013026 and Z141100000214011)
Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Imatinib, Molecular response
Type: Eposter Presentation
Background
Tyrosine kinase inhibitors (TKIs) have become the first line treatment of chronic myeloid leukemia (CML). Because early molecular and cytogenetic responses have been demonstrated to strongly predict long-term outcomes, the 2013 European LeukemiaNet (ELN) recommends that the patient early response should be defined by both molecular and cytogenetic tests to optimally direct the selection of subsequent treatments. The rapid achievement of a deep molecular response has become the treatment goal, therefore, the impact of the combination of early responses on it needs to be investigated.
Aims
We tried to evaluate the impact of the combined molecular and cytogenetic responses at 3 and 6 months on the achievement of molecular response of 4.5 (MR4.5) in imatinib treated CML patients.
Methods
A total of 228 newly diagnosed chronic phase CML patients treated with imatinib were included. At 3 and 6 months, they all were detected BCR-ABL transcript levels by real-time quantitative polymerase chain reaction (RQ-PCR) using peripheral blood samples, and performed karyotyping by standard G-banding techniques. Patients were grouped into 3-month and 6-month molecular optimal cytogenetic optimal (MOCO), molecular optimal cytogenetic warning (MOCW), molecular warning cytogenetic optimal (MWCO), molecular warning cytogenetic warning (MWCW) and failure groups according to the 2013 ELN recommendation. BCR-ABLIS (BCR-ABL levels according to the international scale) was serial followed up for molecular response evaluation.
Results
The median follow-up time was 27 months (range 8-94). For 3 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (8.3% [95% confidence interval (CI), 0-70.5%], 0, 0 and 0, all P>0.05), and they all were significantly lower than that of MOCO patients (35.5% [95% CI, 23.2-48.0%]; P=0.0047, 0.0057, 0.0089 and 0.0081, respectively). Similarly, for 6 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (5.6% [95% CI, 0-66.6%], 20.0% [95% CI, 0.2-67.0%], 0 and 0, all P>0.05), and they were all significantly lower than that of MOCO patients (41.6% [95% CI, 29.2-53.5%]; P=0.025, 0.021, 0.0005 and 0.0010, respectively). Next, responses at 3 and 6 months were combined. Three-month MOCO/6-month non-MOCO, 3-month non-MOCO/6-month MOCO and 3-month non-MOCO/6-month non-MOCO patients had similar 3-year MR4.5 rates (9.4% [95% CI, 0-56.5%], 0 and 4.2% [95% CI, 0-64.0%], P>0.05), and they were all significantly lower than that of 3-month MOCO/6-month MOCO patients (45.4% [95%CI, 32.9-57.1%]; P=0.0024, 0.0073 and <0.0001). This patient group also had significantly higher 3-year event-free survival (EFS) and progression-free survival rates (PFS) than those of patients with non-MOCO at 3 and/or 6 months (P<0.0001 and 0.0065) and had a significantly higher 3-year MR4.5 rate than that of patients with a 3-month/6-month molecularly optimal response but not a cytogenetically optimal response at 3 and/or 6 months (including 3-month MOCO/6-month MOCW, 3-month MOCW/6-month MOCO and 3-month MOCW/6-month MOCW, 5.0% [95%CI, 0-65.6%], P=0.0028).
Conclusion
The combination of early molecular and cytogenetic responses better predicts a deep molecular response in CML patients treated with imatinib. Concurrent optimal molecular and cytogenetic responses at both 3 and 6 months are associated with MR4.5 achievement.Grant support The Nature Science Foundation of China (81370637 and 81570130) and the Beijing Municipal Science and Technology Program (Z131100004013026 and Z141100000214011)

Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Imatinib, Molecular response
Abstract: E1115
Type: Eposter Presentation
Background
Tyrosine kinase inhibitors (TKIs) have become the first line treatment of chronic myeloid leukemia (CML). Because early molecular and cytogenetic responses have been demonstrated to strongly predict long-term outcomes, the 2013 European LeukemiaNet (ELN) recommends that the patient early response should be defined by both molecular and cytogenetic tests to optimally direct the selection of subsequent treatments. The rapid achievement of a deep molecular response has become the treatment goal, therefore, the impact of the combination of early responses on it needs to be investigated.
Aims
We tried to evaluate the impact of the combined molecular and cytogenetic responses at 3 and 6 months on the achievement of molecular response of 4.5 (MR4.5) in imatinib treated CML patients.
Methods
A total of 228 newly diagnosed chronic phase CML patients treated with imatinib were included. At 3 and 6 months, they all were detected BCR-ABL transcript levels by real-time quantitative polymerase chain reaction (RQ-PCR) using peripheral blood samples, and performed karyotyping by standard G-banding techniques. Patients were grouped into 3-month and 6-month molecular optimal cytogenetic optimal (MOCO), molecular optimal cytogenetic warning (MOCW), molecular warning cytogenetic optimal (MWCO), molecular warning cytogenetic warning (MWCW) and failure groups according to the 2013 ELN recommendation. BCR-ABLIS (BCR-ABL levels according to the international scale) was serial followed up for molecular response evaluation.
Results
The median follow-up time was 27 months (range 8-94). For 3 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (8.3% [95% confidence interval (CI), 0-70.5%], 0, 0 and 0, all P>0.05), and they all were significantly lower than that of MOCO patients (35.5% [95% CI, 23.2-48.0%]; P=0.0047, 0.0057, 0.0089 and 0.0081, respectively). Similarly, for 6 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (5.6% [95% CI, 0-66.6%], 20.0% [95% CI, 0.2-67.0%], 0 and 0, all P>0.05), and they were all significantly lower than that of MOCO patients (41.6% [95% CI, 29.2-53.5%]; P=0.025, 0.021, 0.0005 and 0.0010, respectively). Next, responses at 3 and 6 months were combined. Three-month MOCO/6-month non-MOCO, 3-month non-MOCO/6-month MOCO and 3-month non-MOCO/6-month non-MOCO patients had similar 3-year MR4.5 rates (9.4% [95% CI, 0-56.5%], 0 and 4.2% [95% CI, 0-64.0%], P>0.05), and they were all significantly lower than that of 3-month MOCO/6-month MOCO patients (45.4% [95%CI, 32.9-57.1%]; P=0.0024, 0.0073 and <0.0001). This patient group also had significantly higher 3-year event-free survival (EFS) and progression-free survival rates (PFS) than those of patients with non-MOCO at 3 and/or 6 months (P<0.0001 and 0.0065) and had a significantly higher 3-year MR4.5 rate than that of patients with a 3-month/6-month molecularly optimal response but not a cytogenetically optimal response at 3 and/or 6 months (including 3-month MOCO/6-month MOCW, 3-month MOCW/6-month MOCO and 3-month MOCW/6-month MOCW, 5.0% [95%CI, 0-65.6%], P=0.0028).
Conclusion
The combination of early molecular and cytogenetic responses better predicts a deep molecular response in CML patients treated with imatinib. Concurrent optimal molecular and cytogenetic responses at both 3 and 6 months are associated with MR4.5 achievement.Grant support The Nature Science Foundation of China (81370637 and 81570130) and the Beijing Municipal Science and Technology Program (Z131100004013026 and Z141100000214011)

Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Imatinib, Molecular response
Type: Eposter Presentation
Background
Tyrosine kinase inhibitors (TKIs) have become the first line treatment of chronic myeloid leukemia (CML). Because early molecular and cytogenetic responses have been demonstrated to strongly predict long-term outcomes, the 2013 European LeukemiaNet (ELN) recommends that the patient early response should be defined by both molecular and cytogenetic tests to optimally direct the selection of subsequent treatments. The rapid achievement of a deep molecular response has become the treatment goal, therefore, the impact of the combination of early responses on it needs to be investigated.
Aims
We tried to evaluate the impact of the combined molecular and cytogenetic responses at 3 and 6 months on the achievement of molecular response of 4.5 (MR4.5) in imatinib treated CML patients.
Methods
A total of 228 newly diagnosed chronic phase CML patients treated with imatinib were included. At 3 and 6 months, they all were detected BCR-ABL transcript levels by real-time quantitative polymerase chain reaction (RQ-PCR) using peripheral blood samples, and performed karyotyping by standard G-banding techniques. Patients were grouped into 3-month and 6-month molecular optimal cytogenetic optimal (MOCO), molecular optimal cytogenetic warning (MOCW), molecular warning cytogenetic optimal (MWCO), molecular warning cytogenetic warning (MWCW) and failure groups according to the 2013 ELN recommendation. BCR-ABLIS (BCR-ABL levels according to the international scale) was serial followed up for molecular response evaluation.
Results
The median follow-up time was 27 months (range 8-94). For 3 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (8.3% [95% confidence interval (CI), 0-70.5%], 0, 0 and 0, all P>0.05), and they all were significantly lower than that of MOCO patients (35.5% [95% CI, 23.2-48.0%]; P=0.0047, 0.0057, 0.0089 and 0.0081, respectively). Similarly, for 6 months, both MOCW and MWCO patients had 3-year MR4.5 rates similar to those of MWCW and failure patients (5.6% [95% CI, 0-66.6%], 20.0% [95% CI, 0.2-67.0%], 0 and 0, all P>0.05), and they were all significantly lower than that of MOCO patients (41.6% [95% CI, 29.2-53.5%]; P=0.025, 0.021, 0.0005 and 0.0010, respectively). Next, responses at 3 and 6 months were combined. Three-month MOCO/6-month non-MOCO, 3-month non-MOCO/6-month MOCO and 3-month non-MOCO/6-month non-MOCO patients had similar 3-year MR4.5 rates (9.4% [95% CI, 0-56.5%], 0 and 4.2% [95% CI, 0-64.0%], P>0.05), and they were all significantly lower than that of 3-month MOCO/6-month MOCO patients (45.4% [95%CI, 32.9-57.1%]; P=0.0024, 0.0073 and <0.0001). This patient group also had significantly higher 3-year event-free survival (EFS) and progression-free survival rates (PFS) than those of patients with non-MOCO at 3 and/or 6 months (P<0.0001 and 0.0065) and had a significantly higher 3-year MR4.5 rate than that of patients with a 3-month/6-month molecularly optimal response but not a cytogenetically optimal response at 3 and/or 6 months (including 3-month MOCO/6-month MOCW, 3-month MOCW/6-month MOCO and 3-month MOCW/6-month MOCW, 5.0% [95%CI, 0-65.6%], P=0.0028).
Conclusion
The combination of early molecular and cytogenetic responses better predicts a deep molecular response in CML patients treated with imatinib. Concurrent optimal molecular and cytogenetic responses at both 3 and 6 months are associated with MR4.5 achievement.Grant support The Nature Science Foundation of China (81370637 and 81570130) and the Beijing Municipal Science and Technology Program (Z131100004013026 and Z141100000214011)

Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Imatinib, Molecular response
{{ help_message }}
{{filter}}


