IMPACT OF IMATINIB PHARMACOKINETICS ON HEALTH RELATED QUALITY OF LIFE AND ADVERSE EVENTS IN PATIENTS WITH CHRONIC MYELOID LEUKEMIA
(Abstract release date: 05/19/16)
EHA Library. Cao J. 06/09/16; 132660; E1111

Ms. Jinyi Cao
Contributions
Contributions
Abstract
Abstract: E1111
Type: Eposter Presentation
Background
Imatinib has improved survival of chronic myeloid leukemia (CML) patients considerably. However, many patients suffer persistent, low-grade adverse events (AEs) and have relatively inferior health-related quality of life (HRQOL). This impairment is in part ascribed to low grade AEs, which is experienced by a large proportion of patients. Studies have demonstrated that imatinib pharmacokinetics may correlate with imatinib-related AEs but none investigated the impact on HRQOL.
Aims
To determine the correlation of imatinib pharmacokinetics with HRQOL, incidence and severity of AEs.
Methods
A prospective cross-sectional study of 70 chronic phase CML patients at Singapore General Hospital was performed. All patients were on imatinib for at least 6 months. Blood samples for imatinib pharmacokinetic analysis were taken at 0 (pre-dose, trough) and 2 hours (peak) after imatinib administration and analyzed via high performance liquid chromatography. Oral clearance was calculated using: Cloral = (D/2t)/Css, where D = dose (mg), t = dosing interval (hr), and Css = steady state imatinib concentration (ng/ml). AEs and HRQOL were measured using M.D. Anderson Symptom Inventory Chronic Myeloid Leukemia Module (MDASI-CML), which was filled up within 48 hours of imatinib pharmacokinetic sampling. MDASI-CML is a multi-symptom patient-reported outcome comprising of 20 symptom items (13 core MDASI items, 7 CML-specific symptom items) and 6 HRQOL interference items. Higher scores indicate more severe AEs and worse HRQOL. Clinical responses was determined based on European LeukemiaNet 2013 guidelines. The study was approved by the institutional review board and all participants were required to sign informed consent.
Results
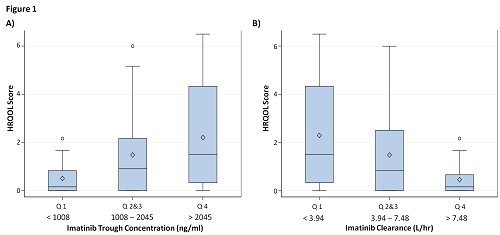
Median daily dose of imatinib was 400mg and the median imatinib trough concentration, peak concentration and clearance were 1365 ng/ml, 2372 ng/ml and 5.74 L/hr respectively. Impairment in at least one of the six HRQOL life activities was experienced by 65.7% of patients while 91.4% of patients experienced at least one AE. Higher HRQOL scores significantly correlated with higher imatinib trough (r = 0.274; p = 0.021) and slower clearance (r = -0.331; p = 0.005) but not with imatinib peak concentrations. Median HRQOL score was significantly higher in the trough Q4 group compared to the Q1 group (1.50 vs 0.17; p = 0.034) and was significantly lower in the clearance Q4 group compared to the Q1 group (0.17 vs 1.50; p = 0.010) (see Figure 1). Higher imatinib trough and slower clearance also correlated with increased patient-rated incidence of AEs (r = 0.238; p = 0.045 and r = -0.278; p = 0.020 respectively) and increased severity of AEs (r = 0.248; p = 0.037 and r = -0.315; p = 0.008 respectively), but not with clinical response.
Conclusion
Higher imatinib trough levels and slower clearance of imatinib are associated with poorer HRQOL, higher incidence and increased severity of AEs. Dose adjustment using imatinib trough levels may be beneficial in reducing AEs and improving HRQOL.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Pharmacokinetic, Quality of life
Type: Eposter Presentation
Background
Imatinib has improved survival of chronic myeloid leukemia (CML) patients considerably. However, many patients suffer persistent, low-grade adverse events (AEs) and have relatively inferior health-related quality of life (HRQOL). This impairment is in part ascribed to low grade AEs, which is experienced by a large proportion of patients. Studies have demonstrated that imatinib pharmacokinetics may correlate with imatinib-related AEs but none investigated the impact on HRQOL.
Aims
To determine the correlation of imatinib pharmacokinetics with HRQOL, incidence and severity of AEs.
Methods
A prospective cross-sectional study of 70 chronic phase CML patients at Singapore General Hospital was performed. All patients were on imatinib for at least 6 months. Blood samples for imatinib pharmacokinetic analysis were taken at 0 (pre-dose, trough) and 2 hours (peak) after imatinib administration and analyzed via high performance liquid chromatography. Oral clearance was calculated using: Cloral = (D/2t)/Css, where D = dose (mg), t = dosing interval (hr), and Css = steady state imatinib concentration (ng/ml). AEs and HRQOL were measured using M.D. Anderson Symptom Inventory Chronic Myeloid Leukemia Module (MDASI-CML), which was filled up within 48 hours of imatinib pharmacokinetic sampling. MDASI-CML is a multi-symptom patient-reported outcome comprising of 20 symptom items (13 core MDASI items, 7 CML-specific symptom items) and 6 HRQOL interference items. Higher scores indicate more severe AEs and worse HRQOL. Clinical responses was determined based on European LeukemiaNet 2013 guidelines. The study was approved by the institutional review board and all participants were required to sign informed consent.
Results
Median daily dose of imatinib was 400mg and the median imatinib trough concentration, peak concentration and clearance were 1365 ng/ml, 2372 ng/ml and 5.74 L/hr respectively. Impairment in at least one of the six HRQOL life activities was experienced by 65.7% of patients while 91.4% of patients experienced at least one AE. Higher HRQOL scores significantly correlated with higher imatinib trough (r = 0.274; p = 0.021) and slower clearance (r = -0.331; p = 0.005) but not with imatinib peak concentrations. Median HRQOL score was significantly higher in the trough Q4 group compared to the Q1 group (1.50 vs 0.17; p = 0.034) and was significantly lower in the clearance Q4 group compared to the Q1 group (0.17 vs 1.50; p = 0.010) (see Figure 1). Higher imatinib trough and slower clearance also correlated with increased patient-rated incidence of AEs (r = 0.238; p = 0.045 and r = -0.278; p = 0.020 respectively) and increased severity of AEs (r = 0.248; p = 0.037 and r = -0.315; p = 0.008 respectively), but not with clinical response.
Conclusion
Higher imatinib trough levels and slower clearance of imatinib are associated with poorer HRQOL, higher incidence and increased severity of AEs. Dose adjustment using imatinib trough levels may be beneficial in reducing AEs and improving HRQOL.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Pharmacokinetic, Quality of life
Abstract: E1111
Type: Eposter Presentation
Background
Imatinib has improved survival of chronic myeloid leukemia (CML) patients considerably. However, many patients suffer persistent, low-grade adverse events (AEs) and have relatively inferior health-related quality of life (HRQOL). This impairment is in part ascribed to low grade AEs, which is experienced by a large proportion of patients. Studies have demonstrated that imatinib pharmacokinetics may correlate with imatinib-related AEs but none investigated the impact on HRQOL.
Aims
To determine the correlation of imatinib pharmacokinetics with HRQOL, incidence and severity of AEs.
Methods
A prospective cross-sectional study of 70 chronic phase CML patients at Singapore General Hospital was performed. All patients were on imatinib for at least 6 months. Blood samples for imatinib pharmacokinetic analysis were taken at 0 (pre-dose, trough) and 2 hours (peak) after imatinib administration and analyzed via high performance liquid chromatography. Oral clearance was calculated using: Cloral = (D/2t)/Css, where D = dose (mg), t = dosing interval (hr), and Css = steady state imatinib concentration (ng/ml). AEs and HRQOL were measured using M.D. Anderson Symptom Inventory Chronic Myeloid Leukemia Module (MDASI-CML), which was filled up within 48 hours of imatinib pharmacokinetic sampling. MDASI-CML is a multi-symptom patient-reported outcome comprising of 20 symptom items (13 core MDASI items, 7 CML-specific symptom items) and 6 HRQOL interference items. Higher scores indicate more severe AEs and worse HRQOL. Clinical responses was determined based on European LeukemiaNet 2013 guidelines. The study was approved by the institutional review board and all participants were required to sign informed consent.
Results
Median daily dose of imatinib was 400mg and the median imatinib trough concentration, peak concentration and clearance were 1365 ng/ml, 2372 ng/ml and 5.74 L/hr respectively. Impairment in at least one of the six HRQOL life activities was experienced by 65.7% of patients while 91.4% of patients experienced at least one AE. Higher HRQOL scores significantly correlated with higher imatinib trough (r = 0.274; p = 0.021) and slower clearance (r = -0.331; p = 0.005) but not with imatinib peak concentrations. Median HRQOL score was significantly higher in the trough Q4 group compared to the Q1 group (1.50 vs 0.17; p = 0.034) and was significantly lower in the clearance Q4 group compared to the Q1 group (0.17 vs 1.50; p = 0.010) (see Figure 1). Higher imatinib trough and slower clearance also correlated with increased patient-rated incidence of AEs (r = 0.238; p = 0.045 and r = -0.278; p = 0.020 respectively) and increased severity of AEs (r = 0.248; p = 0.037 and r = -0.315; p = 0.008 respectively), but not with clinical response.
Conclusion
Higher imatinib trough levels and slower clearance of imatinib are associated with poorer HRQOL, higher incidence and increased severity of AEs. Dose adjustment using imatinib trough levels may be beneficial in reducing AEs and improving HRQOL.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Pharmacokinetic, Quality of life
Type: Eposter Presentation
Background
Imatinib has improved survival of chronic myeloid leukemia (CML) patients considerably. However, many patients suffer persistent, low-grade adverse events (AEs) and have relatively inferior health-related quality of life (HRQOL). This impairment is in part ascribed to low grade AEs, which is experienced by a large proportion of patients. Studies have demonstrated that imatinib pharmacokinetics may correlate with imatinib-related AEs but none investigated the impact on HRQOL.
Aims
To determine the correlation of imatinib pharmacokinetics with HRQOL, incidence and severity of AEs.
Methods
A prospective cross-sectional study of 70 chronic phase CML patients at Singapore General Hospital was performed. All patients were on imatinib for at least 6 months. Blood samples for imatinib pharmacokinetic analysis were taken at 0 (pre-dose, trough) and 2 hours (peak) after imatinib administration and analyzed via high performance liquid chromatography. Oral clearance was calculated using: Cloral = (D/2t)/Css, where D = dose (mg), t = dosing interval (hr), and Css = steady state imatinib concentration (ng/ml). AEs and HRQOL were measured using M.D. Anderson Symptom Inventory Chronic Myeloid Leukemia Module (MDASI-CML), which was filled up within 48 hours of imatinib pharmacokinetic sampling. MDASI-CML is a multi-symptom patient-reported outcome comprising of 20 symptom items (13 core MDASI items, 7 CML-specific symptom items) and 6 HRQOL interference items. Higher scores indicate more severe AEs and worse HRQOL. Clinical responses was determined based on European LeukemiaNet 2013 guidelines. The study was approved by the institutional review board and all participants were required to sign informed consent.
Results
Median daily dose of imatinib was 400mg and the median imatinib trough concentration, peak concentration and clearance were 1365 ng/ml, 2372 ng/ml and 5.74 L/hr respectively. Impairment in at least one of the six HRQOL life activities was experienced by 65.7% of patients while 91.4% of patients experienced at least one AE. Higher HRQOL scores significantly correlated with higher imatinib trough (r = 0.274; p = 0.021) and slower clearance (r = -0.331; p = 0.005) but not with imatinib peak concentrations. Median HRQOL score was significantly higher in the trough Q4 group compared to the Q1 group (1.50 vs 0.17; p = 0.034) and was significantly lower in the clearance Q4 group compared to the Q1 group (0.17 vs 1.50; p = 0.010) (see Figure 1). Higher imatinib trough and slower clearance also correlated with increased patient-rated incidence of AEs (r = 0.238; p = 0.045 and r = -0.278; p = 0.020 respectively) and increased severity of AEs (r = 0.248; p = 0.037 and r = -0.315; p = 0.008 respectively), but not with clinical response.
Conclusion
Higher imatinib trough levels and slower clearance of imatinib are associated with poorer HRQOL, higher incidence and increased severity of AEs. Dose adjustment using imatinib trough levels may be beneficial in reducing AEs and improving HRQOL.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Pharmacokinetic, Quality of life
{{ help_message }}
{{filter}}


