PRELIMINARY FINDINGS FROM A CHART REVIEW OF LOWER DOSING OF PONATINIB IN CHRONIC MYELOID LEUKEMIA (CML) PATIENTS
(Abstract release date: 05/19/16)
EHA Library. Mauro M. 06/09/16; 132659; E1110

Dr. Michael Mauro
Contributions
Contributions
Abstract
Abstract: E1110
Type: Eposter Presentation
Background
Ponatinib is approved for adult patients with refractory CML or Ph+ ALL and those with the T315I mutation. Current prescribing information recommends a starting dose of 45 mg/day, with consideration of lower doses in patients with selected comorbidities, to manage adverse events, and for patients who achieve response. Post hoc dose-response analyses from the registrational PACE trial suggest lower doses of ponatinib may mitigate safety risk while maintaining response; however, outcomes of patients with lower doses have not been evaluated in clinical practice.
Aims
Characterize response and adverse events of special interest (AESI) in real-world CML patients treated with ponatinib doses ≤ 45mg/day.
Methods
We conducted a chart review of adult (aged ≥18 years) US CML patients receiving ponatinib. Patients were required to have ≥6 months of follow-up from first dose; however, no minimum ponatinib exposure was required. Data were abstracted from 6 months prior to first dose (“baseline”) through date of chart review, death or loss to follow up. Baseline characteristics, treatment response and AESI (including arterial occlusive events [AOEs] and venous thromboembolic events) are reported overall and by average daily dose ≤30 mg (“low dose”) and >30 mg (“standard dose”), calculated including therapy gaps as “zero” dose.
Results
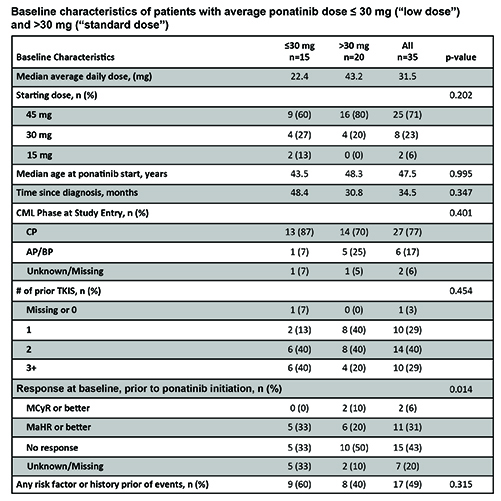
Preliminary analysis of 35 patients from 9 US sites found patients did not differ significantly by dosage group in baseline age, CML phase, time since diagnosis or prior therapy lines; however, fewer low dose patients had no response at start of ponatinib therapy (Table). Significantly more low dose patients had hyperlipidemia at baseline (p=0.027), and there was a trend toward more history of myocardial infarction (MI), coronary artery disease and revascularization among low dose patients (all p=0.070). Low dose patients had nominally more baseline risk factors or prior events compared to standard dose patients (Table). Median time on therapy was 11 vs 12 months for low dose vs standard dose patients, respectively (p=0.336), and dose reduction was common (73% vs 60%; p=0.411). Best response of major molecular response (MMR) or better was reported in 40% of low dose patients and 25% of standard dose (p=0.344); major cytogenetic response (MCyR) or better in 53% vs. 45% (p=0.625), and major hematologic response (MaHR) or better in 80% and 65% of low vs standard dose, respectively (p=0.331). AESIs reported in ≥2 patients were: venous recanalization (n=8), mesenteric artery stent insert (n=6), cerebral small vessel ischemic disease (n=6), MI (n=3), increased troponin (n=3), cardiac arrest (n=2), creatine phosphokinase (n=2). There was no statistically significant difference in AESIs between groups; however, a trend toward more AOEs in patients with lower average doses (p=0.076) was noted, perhaps due to patient selection or post-event dose reduction. Power to detect differences was limited by sample size; further analysis of correlation between baseline risk factors and outcomes is ongoing.
Conclusion
Preliminary data suggest that in clinical practice, response is similar among CML patients treated with low and standard dose ponatinib. Patients with selected risk factors or history of prior event received lower average doses, and the trend toward greater number of AOEs in the low dose group suggests that AOEs may be correlated more with risk than dose. Additional data are needed to fully characterize outcomes of patients treated with ponatinib at doses lower than the currently recommended 45 mg/day.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Dose intensity, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Ponatinib is approved for adult patients with refractory CML or Ph+ ALL and those with the T315I mutation. Current prescribing information recommends a starting dose of 45 mg/day, with consideration of lower doses in patients with selected comorbidities, to manage adverse events, and for patients who achieve response. Post hoc dose-response analyses from the registrational PACE trial suggest lower doses of ponatinib may mitigate safety risk while maintaining response; however, outcomes of patients with lower doses have not been evaluated in clinical practice.
Aims
Characterize response and adverse events of special interest (AESI) in real-world CML patients treated with ponatinib doses ≤ 45mg/day.
Methods
We conducted a chart review of adult (aged ≥18 years) US CML patients receiving ponatinib. Patients were required to have ≥6 months of follow-up from first dose; however, no minimum ponatinib exposure was required. Data were abstracted from 6 months prior to first dose (“baseline”) through date of chart review, death or loss to follow up. Baseline characteristics, treatment response and AESI (including arterial occlusive events [AOEs] and venous thromboembolic events) are reported overall and by average daily dose ≤30 mg (“low dose”) and >30 mg (“standard dose”), calculated including therapy gaps as “zero” dose.
Results
Preliminary analysis of 35 patients from 9 US sites found patients did not differ significantly by dosage group in baseline age, CML phase, time since diagnosis or prior therapy lines; however, fewer low dose patients had no response at start of ponatinib therapy (Table). Significantly more low dose patients had hyperlipidemia at baseline (p=0.027), and there was a trend toward more history of myocardial infarction (MI), coronary artery disease and revascularization among low dose patients (all p=0.070). Low dose patients had nominally more baseline risk factors or prior events compared to standard dose patients (Table). Median time on therapy was 11 vs 12 months for low dose vs standard dose patients, respectively (p=0.336), and dose reduction was common (73% vs 60%; p=0.411). Best response of major molecular response (MMR) or better was reported in 40% of low dose patients and 25% of standard dose (p=0.344); major cytogenetic response (MCyR) or better in 53% vs. 45% (p=0.625), and major hematologic response (MaHR) or better in 80% and 65% of low vs standard dose, respectively (p=0.331). AESIs reported in ≥2 patients were: venous recanalization (n=8), mesenteric artery stent insert (n=6), cerebral small vessel ischemic disease (n=6), MI (n=3), increased troponin (n=3), cardiac arrest (n=2), creatine phosphokinase (n=2). There was no statistically significant difference in AESIs between groups; however, a trend toward more AOEs in patients with lower average doses (p=0.076) was noted, perhaps due to patient selection or post-event dose reduction. Power to detect differences was limited by sample size; further analysis of correlation between baseline risk factors and outcomes is ongoing.
Conclusion
Preliminary data suggest that in clinical practice, response is similar among CML patients treated with low and standard dose ponatinib. Patients with selected risk factors or history of prior event received lower average doses, and the trend toward greater number of AOEs in the low dose group suggests that AOEs may be correlated more with risk than dose. Additional data are needed to fully characterize outcomes of patients treated with ponatinib at doses lower than the currently recommended 45 mg/day.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Dose intensity, Tyrosine kinase inhibitor
Abstract: E1110
Type: Eposter Presentation
Background
Ponatinib is approved for adult patients with refractory CML or Ph+ ALL and those with the T315I mutation. Current prescribing information recommends a starting dose of 45 mg/day, with consideration of lower doses in patients with selected comorbidities, to manage adverse events, and for patients who achieve response. Post hoc dose-response analyses from the registrational PACE trial suggest lower doses of ponatinib may mitigate safety risk while maintaining response; however, outcomes of patients with lower doses have not been evaluated in clinical practice.
Aims
Characterize response and adverse events of special interest (AESI) in real-world CML patients treated with ponatinib doses ≤ 45mg/day.
Methods
We conducted a chart review of adult (aged ≥18 years) US CML patients receiving ponatinib. Patients were required to have ≥6 months of follow-up from first dose; however, no minimum ponatinib exposure was required. Data were abstracted from 6 months prior to first dose (“baseline”) through date of chart review, death or loss to follow up. Baseline characteristics, treatment response and AESI (including arterial occlusive events [AOEs] and venous thromboembolic events) are reported overall and by average daily dose ≤30 mg (“low dose”) and >30 mg (“standard dose”), calculated including therapy gaps as “zero” dose.
Results
Preliminary analysis of 35 patients from 9 US sites found patients did not differ significantly by dosage group in baseline age, CML phase, time since diagnosis or prior therapy lines; however, fewer low dose patients had no response at start of ponatinib therapy (Table). Significantly more low dose patients had hyperlipidemia at baseline (p=0.027), and there was a trend toward more history of myocardial infarction (MI), coronary artery disease and revascularization among low dose patients (all p=0.070). Low dose patients had nominally more baseline risk factors or prior events compared to standard dose patients (Table). Median time on therapy was 11 vs 12 months for low dose vs standard dose patients, respectively (p=0.336), and dose reduction was common (73% vs 60%; p=0.411). Best response of major molecular response (MMR) or better was reported in 40% of low dose patients and 25% of standard dose (p=0.344); major cytogenetic response (MCyR) or better in 53% vs. 45% (p=0.625), and major hematologic response (MaHR) or better in 80% and 65% of low vs standard dose, respectively (p=0.331). AESIs reported in ≥2 patients were: venous recanalization (n=8), mesenteric artery stent insert (n=6), cerebral small vessel ischemic disease (n=6), MI (n=3), increased troponin (n=3), cardiac arrest (n=2), creatine phosphokinase (n=2). There was no statistically significant difference in AESIs between groups; however, a trend toward more AOEs in patients with lower average doses (p=0.076) was noted, perhaps due to patient selection or post-event dose reduction. Power to detect differences was limited by sample size; further analysis of correlation between baseline risk factors and outcomes is ongoing.
Conclusion
Preliminary data suggest that in clinical practice, response is similar among CML patients treated with low and standard dose ponatinib. Patients with selected risk factors or history of prior event received lower average doses, and the trend toward greater number of AOEs in the low dose group suggests that AOEs may be correlated more with risk than dose. Additional data are needed to fully characterize outcomes of patients treated with ponatinib at doses lower than the currently recommended 45 mg/day.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Dose intensity, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Ponatinib is approved for adult patients with refractory CML or Ph+ ALL and those with the T315I mutation. Current prescribing information recommends a starting dose of 45 mg/day, with consideration of lower doses in patients with selected comorbidities, to manage adverse events, and for patients who achieve response. Post hoc dose-response analyses from the registrational PACE trial suggest lower doses of ponatinib may mitigate safety risk while maintaining response; however, outcomes of patients with lower doses have not been evaluated in clinical practice.
Aims
Characterize response and adverse events of special interest (AESI) in real-world CML patients treated with ponatinib doses ≤ 45mg/day.
Methods
We conducted a chart review of adult (aged ≥18 years) US CML patients receiving ponatinib. Patients were required to have ≥6 months of follow-up from first dose; however, no minimum ponatinib exposure was required. Data were abstracted from 6 months prior to first dose (“baseline”) through date of chart review, death or loss to follow up. Baseline characteristics, treatment response and AESI (including arterial occlusive events [AOEs] and venous thromboembolic events) are reported overall and by average daily dose ≤30 mg (“low dose”) and >30 mg (“standard dose”), calculated including therapy gaps as “zero” dose.
Results
Preliminary analysis of 35 patients from 9 US sites found patients did not differ significantly by dosage group in baseline age, CML phase, time since diagnosis or prior therapy lines; however, fewer low dose patients had no response at start of ponatinib therapy (Table). Significantly more low dose patients had hyperlipidemia at baseline (p=0.027), and there was a trend toward more history of myocardial infarction (MI), coronary artery disease and revascularization among low dose patients (all p=0.070). Low dose patients had nominally more baseline risk factors or prior events compared to standard dose patients (Table). Median time on therapy was 11 vs 12 months for low dose vs standard dose patients, respectively (p=0.336), and dose reduction was common (73% vs 60%; p=0.411). Best response of major molecular response (MMR) or better was reported in 40% of low dose patients and 25% of standard dose (p=0.344); major cytogenetic response (MCyR) or better in 53% vs. 45% (p=0.625), and major hematologic response (MaHR) or better in 80% and 65% of low vs standard dose, respectively (p=0.331). AESIs reported in ≥2 patients were: venous recanalization (n=8), mesenteric artery stent insert (n=6), cerebral small vessel ischemic disease (n=6), MI (n=3), increased troponin (n=3), cardiac arrest (n=2), creatine phosphokinase (n=2). There was no statistically significant difference in AESIs between groups; however, a trend toward more AOEs in patients with lower average doses (p=0.076) was noted, perhaps due to patient selection or post-event dose reduction. Power to detect differences was limited by sample size; further analysis of correlation between baseline risk factors and outcomes is ongoing.
Conclusion
Preliminary data suggest that in clinical practice, response is similar among CML patients treated with low and standard dose ponatinib. Patients with selected risk factors or history of prior event received lower average doses, and the trend toward greater number of AOEs in the low dose group suggests that AOEs may be correlated more with risk than dose. Additional data are needed to fully characterize outcomes of patients treated with ponatinib at doses lower than the currently recommended 45 mg/day.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Dose intensity, Tyrosine kinase inhibitor
{{ help_message }}
{{filter}}


