PATIENT-REPORTED OUTCOMES FROM AN OPEN-LABEL SAFETY AND EFFICACY STUDY OF BOSUTINIB IN PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA RESISTANT OR INTOLERANT TO PRIOR THERAPY
(Abstract release date: 05/19/16)
EHA Library. Cortes J. 06/09/16; 132658; E1109

Dr. Jorge Cortes
Contributions
Contributions
Abstract
Abstract: E1109
Type: Eposter Presentation
Background
Bosutinib (BOS) is a tyrosine kinase inhibitor indicated for the treatment of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in adult patients with resistance or intolerance to prior therapy. A 5-year update of the safety and efficacy results from a phase 1/2 study of BOS for Ph+ leukemia resistant/intolerant to prior tyrosine kinase inhibitors (clinicaltrials.gov: NCT00261846) has recently been conducted.
Aims
To assess the long-term health-related quality of life (HRQoL), functioning, and symptoms in patients with chronic phase CML following imatinib resistance or intolerance (chronic phase second line; CP2L) or resistance or intolerance to imatinib plus dasatinib and/or nilotinib (chronic phase third line; CP3L), based on patient-reported outcomes (PROs) from this trial.
Methods
Patients received a starting dose of BOS 500 mg/day and completed the EuroQoL 5 Dimensions (EQ-5D) and the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) questionnaires at screening; weeks 4, 8, and 12; every 12 weeks thereafter; and at the end of treatment visit. Mean and 95% CIs were reported. Informed consent was obtained from all patients. The PRO results at 5 years are presented.
Results
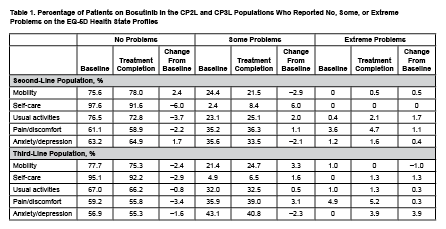
A total of 284 patients were enrolled in the CP2L population and 119 patients in the CP3L population. At treatment completion, EQ-5D completion rates were 67.6% and 65.5% in the CP2L and CP3L populations, respectively; FACT-Leu completion rates were 65.7% and 64.7% in the CP2L and CP3L populations, respectively. At baseline, the mean EQ-5D utility score was 0.83 in the CP2L population and 0.80 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D utility scores ranged from ‒0.04 (‒0.13 to 0.06) to 0.12 (0.03−0.21) in the CP2L population and from −0.05 (−0.16 to 0.07) to 0.01 (‒0.15 to 0.16) in the CP3L population. At baseline, the mean EQ-5D visual analog scale (VAS) score was 71.3 in the CP2L population and 72.6 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D VAS scores ranged from 0 (95% CI not available) to 30.17 (−86.96 to 147.29) in the CP2L population and from ‒2.64 (‒6.14 to 0.85) to 19.69 (−5.30 to 44.68) in the CP3L population. At treatment completion, most patients reported “no problems” on all of the 5 dimensions of the EQ-5D health state profiles (Table 1). At baseline, the mean FACT-General (FACT-G) Total Score and FACT-Leu Total Score were 81.0 and 133.2, respectively, in the CP2L population and 80.8 and 132.5, respectively, in the CP3L population. In the CP2L population, mean (95% CI) changes from baseline in FACT-G Total Score (minimally important difference: 3‒7 points) and FACT-Leu Total Score (minimally important difference: 6‒12 points) ranged from ‒1.02 (‒2.41 to 0.36) to 9.87 (0.94−18.80) and ‒4.66 (‒14.81 to 5.48) to 17.34 (6.33−28.36), respectively. In the CP3L population, mean (95% CI) changes from baseline in FACT-G Total Score and FACT-Leu Total Score ranged from ‒2.74 (‒5.09 to ‒0.39) to 9.50 (95% CI not available) and ‒3.05 (‒6.84 to 0.73) to 7.22 (‒3.40 to 17.85), respectively.
Conclusion
These findings are of considerable importance to patients and physicians and indicate, for the patients who remained in the study, that HRQoL was largely maintained with BOS during the 5-year study duration in both second line and third line patients with Ph+ CML with resistance or intolerance to prior therapy.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Quality of life, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Bosutinib (BOS) is a tyrosine kinase inhibitor indicated for the treatment of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in adult patients with resistance or intolerance to prior therapy. A 5-year update of the safety and efficacy results from a phase 1/2 study of BOS for Ph+ leukemia resistant/intolerant to prior tyrosine kinase inhibitors (clinicaltrials.gov: NCT00261846) has recently been conducted.
Aims
To assess the long-term health-related quality of life (HRQoL), functioning, and symptoms in patients with chronic phase CML following imatinib resistance or intolerance (chronic phase second line; CP2L) or resistance or intolerance to imatinib plus dasatinib and/or nilotinib (chronic phase third line; CP3L), based on patient-reported outcomes (PROs) from this trial.
Methods
Patients received a starting dose of BOS 500 mg/day and completed the EuroQoL 5 Dimensions (EQ-5D) and the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) questionnaires at screening; weeks 4, 8, and 12; every 12 weeks thereafter; and at the end of treatment visit. Mean and 95% CIs were reported. Informed consent was obtained from all patients. The PRO results at 5 years are presented.
Results
A total of 284 patients were enrolled in the CP2L population and 119 patients in the CP3L population. At treatment completion, EQ-5D completion rates were 67.6% and 65.5% in the CP2L and CP3L populations, respectively; FACT-Leu completion rates were 65.7% and 64.7% in the CP2L and CP3L populations, respectively. At baseline, the mean EQ-5D utility score was 0.83 in the CP2L population and 0.80 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D utility scores ranged from ‒0.04 (‒0.13 to 0.06) to 0.12 (0.03−0.21) in the CP2L population and from −0.05 (−0.16 to 0.07) to 0.01 (‒0.15 to 0.16) in the CP3L population. At baseline, the mean EQ-5D visual analog scale (VAS) score was 71.3 in the CP2L population and 72.6 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D VAS scores ranged from 0 (95% CI not available) to 30.17 (−86.96 to 147.29) in the CP2L population and from ‒2.64 (‒6.14 to 0.85) to 19.69 (−5.30 to 44.68) in the CP3L population. At treatment completion, most patients reported “no problems” on all of the 5 dimensions of the EQ-5D health state profiles (Table 1). At baseline, the mean FACT-General (FACT-G) Total Score and FACT-Leu Total Score were 81.0 and 133.2, respectively, in the CP2L population and 80.8 and 132.5, respectively, in the CP3L population. In the CP2L population, mean (95% CI) changes from baseline in FACT-G Total Score (minimally important difference: 3‒7 points) and FACT-Leu Total Score (minimally important difference: 6‒12 points) ranged from ‒1.02 (‒2.41 to 0.36) to 9.87 (0.94−18.80) and ‒4.66 (‒14.81 to 5.48) to 17.34 (6.33−28.36), respectively. In the CP3L population, mean (95% CI) changes from baseline in FACT-G Total Score and FACT-Leu Total Score ranged from ‒2.74 (‒5.09 to ‒0.39) to 9.50 (95% CI not available) and ‒3.05 (‒6.84 to 0.73) to 7.22 (‒3.40 to 17.85), respectively.
Conclusion
These findings are of considerable importance to patients and physicians and indicate, for the patients who remained in the study, that HRQoL was largely maintained with BOS during the 5-year study duration in both second line and third line patients with Ph+ CML with resistance or intolerance to prior therapy.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Quality of life, Tyrosine kinase inhibitor
Abstract: E1109
Type: Eposter Presentation
Background
Bosutinib (BOS) is a tyrosine kinase inhibitor indicated for the treatment of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in adult patients with resistance or intolerance to prior therapy. A 5-year update of the safety and efficacy results from a phase 1/2 study of BOS for Ph+ leukemia resistant/intolerant to prior tyrosine kinase inhibitors (clinicaltrials.gov: NCT00261846) has recently been conducted.
Aims
To assess the long-term health-related quality of life (HRQoL), functioning, and symptoms in patients with chronic phase CML following imatinib resistance or intolerance (chronic phase second line; CP2L) or resistance or intolerance to imatinib plus dasatinib and/or nilotinib (chronic phase third line; CP3L), based on patient-reported outcomes (PROs) from this trial.
Methods
Patients received a starting dose of BOS 500 mg/day and completed the EuroQoL 5 Dimensions (EQ-5D) and the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) questionnaires at screening; weeks 4, 8, and 12; every 12 weeks thereafter; and at the end of treatment visit. Mean and 95% CIs were reported. Informed consent was obtained from all patients. The PRO results at 5 years are presented.
Results
A total of 284 patients were enrolled in the CP2L population and 119 patients in the CP3L population. At treatment completion, EQ-5D completion rates were 67.6% and 65.5% in the CP2L and CP3L populations, respectively; FACT-Leu completion rates were 65.7% and 64.7% in the CP2L and CP3L populations, respectively. At baseline, the mean EQ-5D utility score was 0.83 in the CP2L population and 0.80 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D utility scores ranged from ‒0.04 (‒0.13 to 0.06) to 0.12 (0.03−0.21) in the CP2L population and from −0.05 (−0.16 to 0.07) to 0.01 (‒0.15 to 0.16) in the CP3L population. At baseline, the mean EQ-5D visual analog scale (VAS) score was 71.3 in the CP2L population and 72.6 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D VAS scores ranged from 0 (95% CI not available) to 30.17 (−86.96 to 147.29) in the CP2L population and from ‒2.64 (‒6.14 to 0.85) to 19.69 (−5.30 to 44.68) in the CP3L population. At treatment completion, most patients reported “no problems” on all of the 5 dimensions of the EQ-5D health state profiles (Table 1). At baseline, the mean FACT-General (FACT-G) Total Score and FACT-Leu Total Score were 81.0 and 133.2, respectively, in the CP2L population and 80.8 and 132.5, respectively, in the CP3L population. In the CP2L population, mean (95% CI) changes from baseline in FACT-G Total Score (minimally important difference: 3‒7 points) and FACT-Leu Total Score (minimally important difference: 6‒12 points) ranged from ‒1.02 (‒2.41 to 0.36) to 9.87 (0.94−18.80) and ‒4.66 (‒14.81 to 5.48) to 17.34 (6.33−28.36), respectively. In the CP3L population, mean (95% CI) changes from baseline in FACT-G Total Score and FACT-Leu Total Score ranged from ‒2.74 (‒5.09 to ‒0.39) to 9.50 (95% CI not available) and ‒3.05 (‒6.84 to 0.73) to 7.22 (‒3.40 to 17.85), respectively.
Conclusion
These findings are of considerable importance to patients and physicians and indicate, for the patients who remained in the study, that HRQoL was largely maintained with BOS during the 5-year study duration in both second line and third line patients with Ph+ CML with resistance or intolerance to prior therapy.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Quality of life, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Bosutinib (BOS) is a tyrosine kinase inhibitor indicated for the treatment of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in adult patients with resistance or intolerance to prior therapy. A 5-year update of the safety and efficacy results from a phase 1/2 study of BOS for Ph+ leukemia resistant/intolerant to prior tyrosine kinase inhibitors (clinicaltrials.gov: NCT00261846) has recently been conducted.
Aims
To assess the long-term health-related quality of life (HRQoL), functioning, and symptoms in patients with chronic phase CML following imatinib resistance or intolerance (chronic phase second line; CP2L) or resistance or intolerance to imatinib plus dasatinib and/or nilotinib (chronic phase third line; CP3L), based on patient-reported outcomes (PROs) from this trial.
Methods
Patients received a starting dose of BOS 500 mg/day and completed the EuroQoL 5 Dimensions (EQ-5D) and the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) questionnaires at screening; weeks 4, 8, and 12; every 12 weeks thereafter; and at the end of treatment visit. Mean and 95% CIs were reported. Informed consent was obtained from all patients. The PRO results at 5 years are presented.
Results
A total of 284 patients were enrolled in the CP2L population and 119 patients in the CP3L population. At treatment completion, EQ-5D completion rates were 67.6% and 65.5% in the CP2L and CP3L populations, respectively; FACT-Leu completion rates were 65.7% and 64.7% in the CP2L and CP3L populations, respectively. At baseline, the mean EQ-5D utility score was 0.83 in the CP2L population and 0.80 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D utility scores ranged from ‒0.04 (‒0.13 to 0.06) to 0.12 (0.03−0.21) in the CP2L population and from −0.05 (−0.16 to 0.07) to 0.01 (‒0.15 to 0.16) in the CP3L population. At baseline, the mean EQ-5D visual analog scale (VAS) score was 71.3 in the CP2L population and 72.6 in the CP3L population. Mean (95% CI) changes from baseline in EQ-5D VAS scores ranged from 0 (95% CI not available) to 30.17 (−86.96 to 147.29) in the CP2L population and from ‒2.64 (‒6.14 to 0.85) to 19.69 (−5.30 to 44.68) in the CP3L population. At treatment completion, most patients reported “no problems” on all of the 5 dimensions of the EQ-5D health state profiles (Table 1). At baseline, the mean FACT-General (FACT-G) Total Score and FACT-Leu Total Score were 81.0 and 133.2, respectively, in the CP2L population and 80.8 and 132.5, respectively, in the CP3L population. In the CP2L population, mean (95% CI) changes from baseline in FACT-G Total Score (minimally important difference: 3‒7 points) and FACT-Leu Total Score (minimally important difference: 6‒12 points) ranged from ‒1.02 (‒2.41 to 0.36) to 9.87 (0.94−18.80) and ‒4.66 (‒14.81 to 5.48) to 17.34 (6.33−28.36), respectively. In the CP3L population, mean (95% CI) changes from baseline in FACT-G Total Score and FACT-Leu Total Score ranged from ‒2.74 (‒5.09 to ‒0.39) to 9.50 (95% CI not available) and ‒3.05 (‒6.84 to 0.73) to 7.22 (‒3.40 to 17.85), respectively.
Conclusion
These findings are of considerable importance to patients and physicians and indicate, for the patients who remained in the study, that HRQoL was largely maintained with BOS during the 5-year study duration in both second line and third line patients with Ph+ CML with resistance or intolerance to prior therapy.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Quality of life, Tyrosine kinase inhibitor
{{ help_message }}
{{filter}}


