PLA FLOW; A FLOW CYTOMETRY-BASED ASSAY FOR DETECTION OF BCR-ABL FUSION PROTEIN IN BLOOD CELLS FROM CML PATIENTS
(Abstract release date: 05/19/16)
EHA Library. Löf L. 06/09/16; 132656; E1107

Liza Löf
Contributions
Contributions
Abstract
Abstract: E1107
Type: Eposter Presentation
Background
Chronic myeloid leukemia (CML) is currently diagnosed using RT-PCR and/or FISH to reveal the presence of the fusion mRNA transcripts for BCR-ABL, or of the characteristic Philadelphia chromosome. RT-PCR is also used to monitor the effects of treatment by sensitively measuring transcripts representing minimal residual disease (MRD). It has not been possibly to use flow cytometry to identify the neoplastic cells but such a method would be helpful in the workflow of a hematopathology lab. The PLA flow could provide a strong complement to the powerful RT-PCR method used today, with the advantages of flow cytometry.
Aims
We have now developed a method that successfully detects and enumerates cells harboring the fusion protein BCR-ABL by flow cytometry in CML patients.
Methods
The method, PLAflow, uses the in situ proximity ligation assay (PLA) (Söderberg et al 2006, Leuchowius et al 2009), where two antibodies target the BCR and the ABL part, respectively, of the fusion protein. The antibodies are equipped with DNA oligonucleotides that – when brought in proximity – guide the formation of a circular DNA molecule as a template for localized DNA amplification through rolling circle amplification (RCA). Each RCA-product is then labeled with around 1,500 fluorophore-coupled DNA oligonucleotides, allowing cells to be detected by flow cytometry. Using this method we analyzed blood samples from CML patients and compared it to the routine RT-PCR analysis.
Results
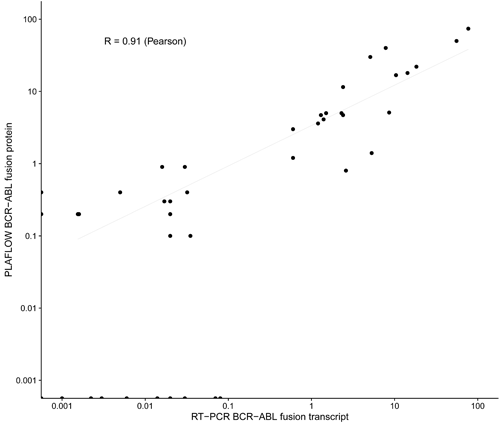
The method that was proven to be very sensitive, enable us to detect very low number of cells harboring BCR-ABL in patient samples. Figure. Blood samples from 47 CML patients analyzed for BCR-ABL positive cells, using FlowPLA (y-axis) and routine analysis of the BCR-ABL transcript using RT-PCR (x-axis) and compared. Pearson correlation coefficient was performed.
Conclusion
The PLA flow is a senstive and robust method that could provide a strong complement to the powerful RT-PCR method used today, both at diagnosis and monitoring MRD.Since the PLA flow method is developed for flow cytometry, and now also adapted for CyTOF, all the advantages with multiparametric analysis can be achieved simultaneously. This allows monitoring of patients using their own cytogenetic signatures, both at diagnosis, and during the MRD monitoring. By investigating which cell populations are expressing the BCR-ABL fusion protein, the method may provide tools to identifying patients responding to a specific treatment.
Session topic: E-poster
Keyword(s): BCR-ABL, Flow cytometry
Type: Eposter Presentation
Background
Chronic myeloid leukemia (CML) is currently diagnosed using RT-PCR and/or FISH to reveal the presence of the fusion mRNA transcripts for BCR-ABL, or of the characteristic Philadelphia chromosome. RT-PCR is also used to monitor the effects of treatment by sensitively measuring transcripts representing minimal residual disease (MRD). It has not been possibly to use flow cytometry to identify the neoplastic cells but such a method would be helpful in the workflow of a hematopathology lab. The PLA flow could provide a strong complement to the powerful RT-PCR method used today, with the advantages of flow cytometry.
Aims
We have now developed a method that successfully detects and enumerates cells harboring the fusion protein BCR-ABL by flow cytometry in CML patients.
Methods
The method, PLAflow, uses the in situ proximity ligation assay (PLA) (Söderberg et al 2006, Leuchowius et al 2009), where two antibodies target the BCR and the ABL part, respectively, of the fusion protein. The antibodies are equipped with DNA oligonucleotides that – when brought in proximity – guide the formation of a circular DNA molecule as a template for localized DNA amplification through rolling circle amplification (RCA). Each RCA-product is then labeled with around 1,500 fluorophore-coupled DNA oligonucleotides, allowing cells to be detected by flow cytometry. Using this method we analyzed blood samples from CML patients and compared it to the routine RT-PCR analysis.
Results
The method that was proven to be very sensitive, enable us to detect very low number of cells harboring BCR-ABL in patient samples. Figure. Blood samples from 47 CML patients analyzed for BCR-ABL positive cells, using FlowPLA (y-axis) and routine analysis of the BCR-ABL transcript using RT-PCR (x-axis) and compared. Pearson correlation coefficient was performed.
Conclusion
The PLA flow is a senstive and robust method that could provide a strong complement to the powerful RT-PCR method used today, both at diagnosis and monitoring MRD.Since the PLA flow method is developed for flow cytometry, and now also adapted for CyTOF, all the advantages with multiparametric analysis can be achieved simultaneously. This allows monitoring of patients using their own cytogenetic signatures, both at diagnosis, and during the MRD monitoring. By investigating which cell populations are expressing the BCR-ABL fusion protein, the method may provide tools to identifying patients responding to a specific treatment.

Session topic: E-poster
Keyword(s): BCR-ABL, Flow cytometry
Abstract: E1107
Type: Eposter Presentation
Background
Chronic myeloid leukemia (CML) is currently diagnosed using RT-PCR and/or FISH to reveal the presence of the fusion mRNA transcripts for BCR-ABL, or of the characteristic Philadelphia chromosome. RT-PCR is also used to monitor the effects of treatment by sensitively measuring transcripts representing minimal residual disease (MRD). It has not been possibly to use flow cytometry to identify the neoplastic cells but such a method would be helpful in the workflow of a hematopathology lab. The PLA flow could provide a strong complement to the powerful RT-PCR method used today, with the advantages of flow cytometry.
Aims
We have now developed a method that successfully detects and enumerates cells harboring the fusion protein BCR-ABL by flow cytometry in CML patients.
Methods
The method, PLAflow, uses the in situ proximity ligation assay (PLA) (Söderberg et al 2006, Leuchowius et al 2009), where two antibodies target the BCR and the ABL part, respectively, of the fusion protein. The antibodies are equipped with DNA oligonucleotides that – when brought in proximity – guide the formation of a circular DNA molecule as a template for localized DNA amplification through rolling circle amplification (RCA). Each RCA-product is then labeled with around 1,500 fluorophore-coupled DNA oligonucleotides, allowing cells to be detected by flow cytometry. Using this method we analyzed blood samples from CML patients and compared it to the routine RT-PCR analysis.
Results
The method that was proven to be very sensitive, enable us to detect very low number of cells harboring BCR-ABL in patient samples. Figure. Blood samples from 47 CML patients analyzed for BCR-ABL positive cells, using FlowPLA (y-axis) and routine analysis of the BCR-ABL transcript using RT-PCR (x-axis) and compared. Pearson correlation coefficient was performed.
Conclusion
The PLA flow is a senstive and robust method that could provide a strong complement to the powerful RT-PCR method used today, both at diagnosis and monitoring MRD.Since the PLA flow method is developed for flow cytometry, and now also adapted for CyTOF, all the advantages with multiparametric analysis can be achieved simultaneously. This allows monitoring of patients using their own cytogenetic signatures, both at diagnosis, and during the MRD monitoring. By investigating which cell populations are expressing the BCR-ABL fusion protein, the method may provide tools to identifying patients responding to a specific treatment.

Session topic: E-poster
Keyword(s): BCR-ABL, Flow cytometry
Type: Eposter Presentation
Background
Chronic myeloid leukemia (CML) is currently diagnosed using RT-PCR and/or FISH to reveal the presence of the fusion mRNA transcripts for BCR-ABL, or of the characteristic Philadelphia chromosome. RT-PCR is also used to monitor the effects of treatment by sensitively measuring transcripts representing minimal residual disease (MRD). It has not been possibly to use flow cytometry to identify the neoplastic cells but such a method would be helpful in the workflow of a hematopathology lab. The PLA flow could provide a strong complement to the powerful RT-PCR method used today, with the advantages of flow cytometry.
Aims
We have now developed a method that successfully detects and enumerates cells harboring the fusion protein BCR-ABL by flow cytometry in CML patients.
Methods
The method, PLAflow, uses the in situ proximity ligation assay (PLA) (Söderberg et al 2006, Leuchowius et al 2009), where two antibodies target the BCR and the ABL part, respectively, of the fusion protein. The antibodies are equipped with DNA oligonucleotides that – when brought in proximity – guide the formation of a circular DNA molecule as a template for localized DNA amplification through rolling circle amplification (RCA). Each RCA-product is then labeled with around 1,500 fluorophore-coupled DNA oligonucleotides, allowing cells to be detected by flow cytometry. Using this method we analyzed blood samples from CML patients and compared it to the routine RT-PCR analysis.
Results
The method that was proven to be very sensitive, enable us to detect very low number of cells harboring BCR-ABL in patient samples. Figure. Blood samples from 47 CML patients analyzed for BCR-ABL positive cells, using FlowPLA (y-axis) and routine analysis of the BCR-ABL transcript using RT-PCR (x-axis) and compared. Pearson correlation coefficient was performed.
Conclusion
The PLA flow is a senstive and robust method that could provide a strong complement to the powerful RT-PCR method used today, both at diagnosis and monitoring MRD.Since the PLA flow method is developed for flow cytometry, and now also adapted for CyTOF, all the advantages with multiparametric analysis can be achieved simultaneously. This allows monitoring of patients using their own cytogenetic signatures, both at diagnosis, and during the MRD monitoring. By investigating which cell populations are expressing the BCR-ABL fusion protein, the method may provide tools to identifying patients responding to a specific treatment.

Session topic: E-poster
Keyword(s): BCR-ABL, Flow cytometry
{{ help_message }}
{{filter}}


