IMATINIB FIRST-LINE WITH SWITCH TO 2ND GENERATION TYROSINE KINASE INHIBITORS IN CASE OF FAILURE OR TOXICITY: REAL-LIFE DATA FROM A POPULATION-BASED REGISTRY OF BCR-ABL1 + CML PATIENTS
(Abstract release date: 05/19/16)
EHA Library. Castagnetti F. 06/09/16; 132654; E1105
Disclosure(s): Consultancy and Honoraria: Novartis, Bristol Myers Squibb, ARIAD, Pfizer

Dr. Fausto Castagnetti
Contributions
Contributions
Abstract
Abstract: E1105
Type: Eposter Presentation
Background
The main academic-and company-sponsored studies of the treatment of CML with TKIs focus on the results achieved with only one TKI, in single-arm trials or in randomized trials comparing two TKIs as first line treatment. After the discontinuation of the study drug, few data on further treatments are collected. In real life and in many countries, for several years imatinib has been used as first-line treatment, but nilotinib and dasatinib have been available and used as second-line therapy.
Aims
To investigate the response and the outcome on first-line imatinib, with switch to 2nd generation tyrosine kinase inhibitors (TKIs) in case of failure or toxicity, in BCR-ABL1+ chronic myeloid leukemia (CML) patients enrolled in a population-based registry (real-life setting).
Methods
From a cohort of 337 consecutive, unselected, newly diagnosed, adult, chronic phase, Ph+, BCR-ABL1+, CML patients who were registered according to population-based criteria in two Italian Regions (Emilia-Romagna and Sicily) between 2008 and 2012, we identified 236 patients who were treated with imatinib 400 mg once daily in first-line. The decision to switch to second-line treatment was up to the Local Investigator (no predefined criteria) Definitions: major molecular response (MMR): BCR-ABL1IS ratio <0.1%; MR4.0: BCR-ABL1IS ratio <0.01% with >10,000 ABL1 copies; failure and suboptimal response: according to 2009 European LeukemiaNet (ELN) criteria; progression: transformation to advanced phases according to ELN criteria; leukemia-related death (LRD): death after progression.
Results
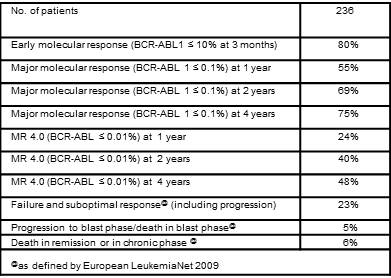
Sokal risk distribution was 29% low, 47% intermediate, and 24% high. Median age was 60 years at diagnosis and 64 years at last analysis, with a median follow up of 4 years. 145 patients (61%) received only imatinib, 57 (24%) were switched to 2nd generation TKIs for failure, and 34 (14%) were switched to 2nd generation TKIs (n=31) or to Hydroxyurea (n=3) for toxicity. The median time to switch was 20 months for failure and 8 months for toxicity. After the switch, the molecular response improved of 1 to 3 logs in 57% of patients. Molecular responses and outcomes are shown in the Table. Eleven patients (5%) progressed to blast phase, and all of them died, but one. The 5-year leukemia-related survival was 95%. The 5-year overall survival was 89%, with 6% of patients dying in major molecular remission or in chronic phase, without any evidence of progression. Noticeably, 48% of living patients had achieved MR 4.0 (BCR-ABL 1 ≤ 0.01% IS) by 4 years (including first- and second-line treatment).
Conclusion
A policy of imatinib first-line with switch to 2nd generation TKIs for failure or toxicity, as recommended by European LeukemiaNet 2009, in an unselected (median age 60 years) cohort of patients registered according to population-based criteria showed a high efficacy.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib resistance, Population, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
The main academic-and company-sponsored studies of the treatment of CML with TKIs focus on the results achieved with only one TKI, in single-arm trials or in randomized trials comparing two TKIs as first line treatment. After the discontinuation of the study drug, few data on further treatments are collected. In real life and in many countries, for several years imatinib has been used as first-line treatment, but nilotinib and dasatinib have been available and used as second-line therapy.
Aims
To investigate the response and the outcome on first-line imatinib, with switch to 2nd generation tyrosine kinase inhibitors (TKIs) in case of failure or toxicity, in BCR-ABL1+ chronic myeloid leukemia (CML) patients enrolled in a population-based registry (real-life setting).
Methods
From a cohort of 337 consecutive, unselected, newly diagnosed, adult, chronic phase, Ph+, BCR-ABL1+, CML patients who were registered according to population-based criteria in two Italian Regions (Emilia-Romagna and Sicily) between 2008 and 2012, we identified 236 patients who were treated with imatinib 400 mg once daily in first-line. The decision to switch to second-line treatment was up to the Local Investigator (no predefined criteria) Definitions: major molecular response (MMR): BCR-ABL1IS ratio <0.1%; MR4.0: BCR-ABL1IS ratio <0.01% with >10,000 ABL1 copies; failure and suboptimal response: according to 2009 European LeukemiaNet (ELN) criteria; progression: transformation to advanced phases according to ELN criteria; leukemia-related death (LRD): death after progression.
Results
Sokal risk distribution was 29% low, 47% intermediate, and 24% high. Median age was 60 years at diagnosis and 64 years at last analysis, with a median follow up of 4 years. 145 patients (61%) received only imatinib, 57 (24%) were switched to 2nd generation TKIs for failure, and 34 (14%) were switched to 2nd generation TKIs (n=31) or to Hydroxyurea (n=3) for toxicity. The median time to switch was 20 months for failure and 8 months for toxicity. After the switch, the molecular response improved of 1 to 3 logs in 57% of patients. Molecular responses and outcomes are shown in the Table. Eleven patients (5%) progressed to blast phase, and all of them died, but one. The 5-year leukemia-related survival was 95%. The 5-year overall survival was 89%, with 6% of patients dying in major molecular remission or in chronic phase, without any evidence of progression. Noticeably, 48% of living patients had achieved MR 4.0 (BCR-ABL 1 ≤ 0.01% IS) by 4 years (including first- and second-line treatment).
Conclusion
A policy of imatinib first-line with switch to 2nd generation TKIs for failure or toxicity, as recommended by European LeukemiaNet 2009, in an unselected (median age 60 years) cohort of patients registered according to population-based criteria showed a high efficacy.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib resistance, Population, Tyrosine kinase inhibitor
Abstract: E1105
Type: Eposter Presentation
Background
The main academic-and company-sponsored studies of the treatment of CML with TKIs focus on the results achieved with only one TKI, in single-arm trials or in randomized trials comparing two TKIs as first line treatment. After the discontinuation of the study drug, few data on further treatments are collected. In real life and in many countries, for several years imatinib has been used as first-line treatment, but nilotinib and dasatinib have been available and used as second-line therapy.
Aims
To investigate the response and the outcome on first-line imatinib, with switch to 2nd generation tyrosine kinase inhibitors (TKIs) in case of failure or toxicity, in BCR-ABL1+ chronic myeloid leukemia (CML) patients enrolled in a population-based registry (real-life setting).
Methods
From a cohort of 337 consecutive, unselected, newly diagnosed, adult, chronic phase, Ph+, BCR-ABL1+, CML patients who were registered according to population-based criteria in two Italian Regions (Emilia-Romagna and Sicily) between 2008 and 2012, we identified 236 patients who were treated with imatinib 400 mg once daily in first-line. The decision to switch to second-line treatment was up to the Local Investigator (no predefined criteria) Definitions: major molecular response (MMR): BCR-ABL1IS ratio <0.1%; MR4.0: BCR-ABL1IS ratio <0.01% with >10,000 ABL1 copies; failure and suboptimal response: according to 2009 European LeukemiaNet (ELN) criteria; progression: transformation to advanced phases according to ELN criteria; leukemia-related death (LRD): death after progression.
Results
Sokal risk distribution was 29% low, 47% intermediate, and 24% high. Median age was 60 years at diagnosis and 64 years at last analysis, with a median follow up of 4 years. 145 patients (61%) received only imatinib, 57 (24%) were switched to 2nd generation TKIs for failure, and 34 (14%) were switched to 2nd generation TKIs (n=31) or to Hydroxyurea (n=3) for toxicity. The median time to switch was 20 months for failure and 8 months for toxicity. After the switch, the molecular response improved of 1 to 3 logs in 57% of patients. Molecular responses and outcomes are shown in the Table. Eleven patients (5%) progressed to blast phase, and all of them died, but one. The 5-year leukemia-related survival was 95%. The 5-year overall survival was 89%, with 6% of patients dying in major molecular remission or in chronic phase, without any evidence of progression. Noticeably, 48% of living patients had achieved MR 4.0 (BCR-ABL 1 ≤ 0.01% IS) by 4 years (including first- and second-line treatment).
Conclusion
A policy of imatinib first-line with switch to 2nd generation TKIs for failure or toxicity, as recommended by European LeukemiaNet 2009, in an unselected (median age 60 years) cohort of patients registered according to population-based criteria showed a high efficacy.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib resistance, Population, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
The main academic-and company-sponsored studies of the treatment of CML with TKIs focus on the results achieved with only one TKI, in single-arm trials or in randomized trials comparing two TKIs as first line treatment. After the discontinuation of the study drug, few data on further treatments are collected. In real life and in many countries, for several years imatinib has been used as first-line treatment, but nilotinib and dasatinib have been available and used as second-line therapy.
Aims
To investigate the response and the outcome on first-line imatinib, with switch to 2nd generation tyrosine kinase inhibitors (TKIs) in case of failure or toxicity, in BCR-ABL1+ chronic myeloid leukemia (CML) patients enrolled in a population-based registry (real-life setting).
Methods
From a cohort of 337 consecutive, unselected, newly diagnosed, adult, chronic phase, Ph+, BCR-ABL1+, CML patients who were registered according to population-based criteria in two Italian Regions (Emilia-Romagna and Sicily) between 2008 and 2012, we identified 236 patients who were treated with imatinib 400 mg once daily in first-line. The decision to switch to second-line treatment was up to the Local Investigator (no predefined criteria) Definitions: major molecular response (MMR): BCR-ABL1IS ratio <0.1%; MR4.0: BCR-ABL1IS ratio <0.01% with >10,000 ABL1 copies; failure and suboptimal response: according to 2009 European LeukemiaNet (ELN) criteria; progression: transformation to advanced phases according to ELN criteria; leukemia-related death (LRD): death after progression.
Results
Sokal risk distribution was 29% low, 47% intermediate, and 24% high. Median age was 60 years at diagnosis and 64 years at last analysis, with a median follow up of 4 years. 145 patients (61%) received only imatinib, 57 (24%) were switched to 2nd generation TKIs for failure, and 34 (14%) were switched to 2nd generation TKIs (n=31) or to Hydroxyurea (n=3) for toxicity. The median time to switch was 20 months for failure and 8 months for toxicity. After the switch, the molecular response improved of 1 to 3 logs in 57% of patients. Molecular responses and outcomes are shown in the Table. Eleven patients (5%) progressed to blast phase, and all of them died, but one. The 5-year leukemia-related survival was 95%. The 5-year overall survival was 89%, with 6% of patients dying in major molecular remission or in chronic phase, without any evidence of progression. Noticeably, 48% of living patients had achieved MR 4.0 (BCR-ABL 1 ≤ 0.01% IS) by 4 years (including first- and second-line treatment).
Conclusion
A policy of imatinib first-line with switch to 2nd generation TKIs for failure or toxicity, as recommended by European LeukemiaNet 2009, in an unselected (median age 60 years) cohort of patients registered according to population-based criteria showed a high efficacy.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib resistance, Population, Tyrosine kinase inhibitor
{{ help_message }}
{{filter}}


