PONATINIB EVALUATION AND SAFETY IN REAL LIFE CHRONIC PHASE (CP) CML PATIENTS FAILING ≥2 TYROSINE KINASE INHIBITORS (TKI): UPDATE OF THE PEARL OBSERVATIONAL STUDY
(Abstract release date: 05/19/16)
EHA Library. Nicolini F. 06/09/16; 132653; E1104

Dr. Franck Nicolini
Contributions
Contributions
Abstract
Abstract: E1104
Type: Eposter Presentation
Background
Ponatinib (PON) induces high response rates in heavily pre-treated CML patients (pts) failing TKIs. A substantial proportion of pts on PON [17% by 3 years, J. Cortes, Haematologica 2015] experience severe arterial thrombotic events (ATE). The impact in the real-life setting of this recently licensed agent is unknown yet.
Aims
We analyzed the French PON compassionate use program (19/04/2012-30/09/2013) to evaluate outcomes in the real-life setting.
Methods
Multicenter observational retrospective study to examine safety and efficacy of PON in CML failing TKIs, benefiting from the national PON compassionate use program. Data collection followed the regulations of observational studies in France. Pts were analyzed in intention-to-treat. Molecular biology tests were expressed as BCR-ABL/ABL (%IS) in all centers. Standard clinical data, cardiovascular risk factors (CVRF), onset of any CV events (before and during PON treatment) and metabolic biological parameters were captured.
Results
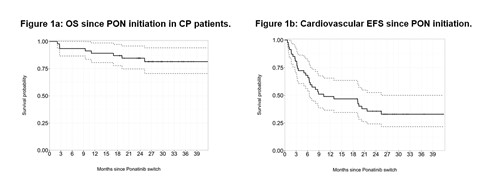
48 patient records were collected in CP, 5 in AP and 6 in BC. We focused our analysis on CP pts only. There were 24 (50%) males, median age of 53 (18-76) years at CML diagnosis and 60 (20-82) years at PON initiation. Sokal scores were high in 15 (31%), intermediate in 17 (35%), low in 6 (12%) and unknown in 10 (22%) pts. Fifteen pts (31%) were treated for hypertension, 2 were diabetic, 11 (23%) had dyslipidemia (all on statins), tobacco abuse was present in 19 (39%) pts (1 unknown) and 21 (44%) pts had some pre-existing cardiovascular risk factors (CVRF) in total prior to PON. Median weight was 66 (50-107) kg, and BMI was 24 (17.85-43) kg/m2. Fifteen (31%) pts (3 unknown) were on anti-aggregants or anti-coagulants (AAG/C) before PON. Two pts had nilotinib, 4 dasatinib and all other pts had imatinib first-line for a median of 22 (2-132) months, 25 (52%) dasatinib and 20 (42)% nilotinib for 14 (0.5-64) months, as second-line, 4 pts developed a T315I mutation after imatinib only; 29 (60%) had received all 3 TKIs prior to PON. At PON initiation, 11 (23%) harbored a T315I mutation, 9 (19%) other mutation(s), 24 (50%) none (3 not done). The trigger for PON was resistance in 34 (71%) pts, intolerance in 11 (23%) pts and both in 3 (6%) pts. Pts were initiated at a median of 45 (15-45) mg daily after a median of 73.5 (17-217) months of disease duration. The median follow-up was 26.5 (2-42) months since PON initiation, and 102.5 (27-230) months since diagnosis. Median time on PON was 19 (0.13-41.79) months. Eight pts died, 6 of disease progression, 1 of myocardial infarction and 1 of PAOD-related complications. The probability of OS was 81.5 (70.5-94)% at 36 months (Fig. 1a) with no influence of the presence of mutations or reason for PON prescription. Overall, the cumulative incidence of MMR was 55 (35-73)% at 18 months. Seven (14.5%) pts had hematologic AEs imposing transient PON withhold, and 19 (40%) diverse grade 1-2 non-hematologic, non-CV AEs (pancreatic, hepatic, skin toxicities, no grade 3-4). Significant CV AEs (including hypertension) occurred in 29 (60%) pts after a median of 5.8 (0.1-25.4) months of PON (Fig 1b), in 11 pts without CVRF. Eight thrombotic events occurred (3 heart strokes, 2 brain strokes, 1 PAOD, 2 DVT). Of note, 3 thrombotic AEs occurred on AAG/C. Lipids and HbA1c do not seem to be modified on PON. At last follow-up 22 (46%) pts are still on PON.
Conclusion
In this real-life setting, CP-CML patients resistant or intolerant to previous TKIs, PON still displayed strong efficacy, ATEs represent the main adverse factor observed.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Ponatinib (PON) induces high response rates in heavily pre-treated CML patients (pts) failing TKIs. A substantial proportion of pts on PON [17% by 3 years, J. Cortes, Haematologica 2015] experience severe arterial thrombotic events (ATE). The impact in the real-life setting of this recently licensed agent is unknown yet.
Aims
We analyzed the French PON compassionate use program (19/04/2012-30/09/2013) to evaluate outcomes in the real-life setting.
Methods
Multicenter observational retrospective study to examine safety and efficacy of PON in CML failing TKIs, benefiting from the national PON compassionate use program. Data collection followed the regulations of observational studies in France. Pts were analyzed in intention-to-treat. Molecular biology tests were expressed as BCR-ABL/ABL (%IS) in all centers. Standard clinical data, cardiovascular risk factors (CVRF), onset of any CV events (before and during PON treatment) and metabolic biological parameters were captured.
Results
48 patient records were collected in CP, 5 in AP and 6 in BC. We focused our analysis on CP pts only. There were 24 (50%) males, median age of 53 (18-76) years at CML diagnosis and 60 (20-82) years at PON initiation. Sokal scores were high in 15 (31%), intermediate in 17 (35%), low in 6 (12%) and unknown in 10 (22%) pts. Fifteen pts (31%) were treated for hypertension, 2 were diabetic, 11 (23%) had dyslipidemia (all on statins), tobacco abuse was present in 19 (39%) pts (1 unknown) and 21 (44%) pts had some pre-existing cardiovascular risk factors (CVRF) in total prior to PON. Median weight was 66 (50-107) kg, and BMI was 24 (17.85-43) kg/m2. Fifteen (31%) pts (3 unknown) were on anti-aggregants or anti-coagulants (AAG/C) before PON. Two pts had nilotinib, 4 dasatinib and all other pts had imatinib first-line for a median of 22 (2-132) months, 25 (52%) dasatinib and 20 (42)% nilotinib for 14 (0.5-64) months, as second-line, 4 pts developed a T315I mutation after imatinib only; 29 (60%) had received all 3 TKIs prior to PON. At PON initiation, 11 (23%) harbored a T315I mutation, 9 (19%) other mutation(s), 24 (50%) none (3 not done). The trigger for PON was resistance in 34 (71%) pts, intolerance in 11 (23%) pts and both in 3 (6%) pts. Pts were initiated at a median of 45 (15-45) mg daily after a median of 73.5 (17-217) months of disease duration. The median follow-up was 26.5 (2-42) months since PON initiation, and 102.5 (27-230) months since diagnosis. Median time on PON was 19 (0.13-41.79) months. Eight pts died, 6 of disease progression, 1 of myocardial infarction and 1 of PAOD-related complications. The probability of OS was 81.5 (70.5-94)% at 36 months (Fig. 1a) with no influence of the presence of mutations or reason for PON prescription. Overall, the cumulative incidence of MMR was 55 (35-73)% at 18 months. Seven (14.5%) pts had hematologic AEs imposing transient PON withhold, and 19 (40%) diverse grade 1-2 non-hematologic, non-CV AEs (pancreatic, hepatic, skin toxicities, no grade 3-4). Significant CV AEs (including hypertension) occurred in 29 (60%) pts after a median of 5.8 (0.1-25.4) months of PON (Fig 1b), in 11 pts without CVRF. Eight thrombotic events occurred (3 heart strokes, 2 brain strokes, 1 PAOD, 2 DVT). Of note, 3 thrombotic AEs occurred on AAG/C. Lipids and HbA1c do not seem to be modified on PON. At last follow-up 22 (46%) pts are still on PON.
Conclusion
In this real-life setting, CP-CML patients resistant or intolerant to previous TKIs, PON still displayed strong efficacy, ATEs represent the main adverse factor observed.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor
Abstract: E1104
Type: Eposter Presentation
Background
Ponatinib (PON) induces high response rates in heavily pre-treated CML patients (pts) failing TKIs. A substantial proportion of pts on PON [17% by 3 years, J. Cortes, Haematologica 2015] experience severe arterial thrombotic events (ATE). The impact in the real-life setting of this recently licensed agent is unknown yet.
Aims
We analyzed the French PON compassionate use program (19/04/2012-30/09/2013) to evaluate outcomes in the real-life setting.
Methods
Multicenter observational retrospective study to examine safety and efficacy of PON in CML failing TKIs, benefiting from the national PON compassionate use program. Data collection followed the regulations of observational studies in France. Pts were analyzed in intention-to-treat. Molecular biology tests were expressed as BCR-ABL/ABL (%IS) in all centers. Standard clinical data, cardiovascular risk factors (CVRF), onset of any CV events (before and during PON treatment) and metabolic biological parameters were captured.
Results
48 patient records were collected in CP, 5 in AP and 6 in BC. We focused our analysis on CP pts only. There were 24 (50%) males, median age of 53 (18-76) years at CML diagnosis and 60 (20-82) years at PON initiation. Sokal scores were high in 15 (31%), intermediate in 17 (35%), low in 6 (12%) and unknown in 10 (22%) pts. Fifteen pts (31%) were treated for hypertension, 2 were diabetic, 11 (23%) had dyslipidemia (all on statins), tobacco abuse was present in 19 (39%) pts (1 unknown) and 21 (44%) pts had some pre-existing cardiovascular risk factors (CVRF) in total prior to PON. Median weight was 66 (50-107) kg, and BMI was 24 (17.85-43) kg/m2. Fifteen (31%) pts (3 unknown) were on anti-aggregants or anti-coagulants (AAG/C) before PON. Two pts had nilotinib, 4 dasatinib and all other pts had imatinib first-line for a median of 22 (2-132) months, 25 (52%) dasatinib and 20 (42)% nilotinib for 14 (0.5-64) months, as second-line, 4 pts developed a T315I mutation after imatinib only; 29 (60%) had received all 3 TKIs prior to PON. At PON initiation, 11 (23%) harbored a T315I mutation, 9 (19%) other mutation(s), 24 (50%) none (3 not done). The trigger for PON was resistance in 34 (71%) pts, intolerance in 11 (23%) pts and both in 3 (6%) pts. Pts were initiated at a median of 45 (15-45) mg daily after a median of 73.5 (17-217) months of disease duration. The median follow-up was 26.5 (2-42) months since PON initiation, and 102.5 (27-230) months since diagnosis. Median time on PON was 19 (0.13-41.79) months. Eight pts died, 6 of disease progression, 1 of myocardial infarction and 1 of PAOD-related complications. The probability of OS was 81.5 (70.5-94)% at 36 months (Fig. 1a) with no influence of the presence of mutations or reason for PON prescription. Overall, the cumulative incidence of MMR was 55 (35-73)% at 18 months. Seven (14.5%) pts had hematologic AEs imposing transient PON withhold, and 19 (40%) diverse grade 1-2 non-hematologic, non-CV AEs (pancreatic, hepatic, skin toxicities, no grade 3-4). Significant CV AEs (including hypertension) occurred in 29 (60%) pts after a median of 5.8 (0.1-25.4) months of PON (Fig 1b), in 11 pts without CVRF. Eight thrombotic events occurred (3 heart strokes, 2 brain strokes, 1 PAOD, 2 DVT). Of note, 3 thrombotic AEs occurred on AAG/C. Lipids and HbA1c do not seem to be modified on PON. At last follow-up 22 (46%) pts are still on PON.
Conclusion
In this real-life setting, CP-CML patients resistant or intolerant to previous TKIs, PON still displayed strong efficacy, ATEs represent the main adverse factor observed.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Ponatinib (PON) induces high response rates in heavily pre-treated CML patients (pts) failing TKIs. A substantial proportion of pts on PON [17% by 3 years, J. Cortes, Haematologica 2015] experience severe arterial thrombotic events (ATE). The impact in the real-life setting of this recently licensed agent is unknown yet.
Aims
We analyzed the French PON compassionate use program (19/04/2012-30/09/2013) to evaluate outcomes in the real-life setting.
Methods
Multicenter observational retrospective study to examine safety and efficacy of PON in CML failing TKIs, benefiting from the national PON compassionate use program. Data collection followed the regulations of observational studies in France. Pts were analyzed in intention-to-treat. Molecular biology tests were expressed as BCR-ABL/ABL (%IS) in all centers. Standard clinical data, cardiovascular risk factors (CVRF), onset of any CV events (before and during PON treatment) and metabolic biological parameters were captured.
Results
48 patient records were collected in CP, 5 in AP and 6 in BC. We focused our analysis on CP pts only. There were 24 (50%) males, median age of 53 (18-76) years at CML diagnosis and 60 (20-82) years at PON initiation. Sokal scores were high in 15 (31%), intermediate in 17 (35%), low in 6 (12%) and unknown in 10 (22%) pts. Fifteen pts (31%) were treated for hypertension, 2 were diabetic, 11 (23%) had dyslipidemia (all on statins), tobacco abuse was present in 19 (39%) pts (1 unknown) and 21 (44%) pts had some pre-existing cardiovascular risk factors (CVRF) in total prior to PON. Median weight was 66 (50-107) kg, and BMI was 24 (17.85-43) kg/m2. Fifteen (31%) pts (3 unknown) were on anti-aggregants or anti-coagulants (AAG/C) before PON. Two pts had nilotinib, 4 dasatinib and all other pts had imatinib first-line for a median of 22 (2-132) months, 25 (52%) dasatinib and 20 (42)% nilotinib for 14 (0.5-64) months, as second-line, 4 pts developed a T315I mutation after imatinib only; 29 (60%) had received all 3 TKIs prior to PON. At PON initiation, 11 (23%) harbored a T315I mutation, 9 (19%) other mutation(s), 24 (50%) none (3 not done). The trigger for PON was resistance in 34 (71%) pts, intolerance in 11 (23%) pts and both in 3 (6%) pts. Pts were initiated at a median of 45 (15-45) mg daily after a median of 73.5 (17-217) months of disease duration. The median follow-up was 26.5 (2-42) months since PON initiation, and 102.5 (27-230) months since diagnosis. Median time on PON was 19 (0.13-41.79) months. Eight pts died, 6 of disease progression, 1 of myocardial infarction and 1 of PAOD-related complications. The probability of OS was 81.5 (70.5-94)% at 36 months (Fig. 1a) with no influence of the presence of mutations or reason for PON prescription. Overall, the cumulative incidence of MMR was 55 (35-73)% at 18 months. Seven (14.5%) pts had hematologic AEs imposing transient PON withhold, and 19 (40%) diverse grade 1-2 non-hematologic, non-CV AEs (pancreatic, hepatic, skin toxicities, no grade 3-4). Significant CV AEs (including hypertension) occurred in 29 (60%) pts after a median of 5.8 (0.1-25.4) months of PON (Fig 1b), in 11 pts without CVRF. Eight thrombotic events occurred (3 heart strokes, 2 brain strokes, 1 PAOD, 2 DVT). Of note, 3 thrombotic AEs occurred on AAG/C. Lipids and HbA1c do not seem to be modified on PON. At last follow-up 22 (46%) pts are still on PON.
Conclusion
In this real-life setting, CP-CML patients resistant or intolerant to previous TKIs, PON still displayed strong efficacy, ATEs represent the main adverse factor observed.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor
{{ help_message }}
{{filter}}


