MEASUREMENT OF BCR-ABL1 BY RT-QPCR IN CHRONIC MYELOID LEUKAEMIA: FINDINGS FROM AN INTERNATIONAL EQA PROGRAMME
(Abstract release date: 05/19/16)
EHA Library. Scott S. 06/09/16; 132651; E1102

Mr. Stuart Scott
Contributions
Contributions
Abstract
Abstract: E1102
Type: Eposter Presentation
Background
BCR-ABL1 measurement by RT-qPCR is integral to both the diagnosis and monitoring of Chronic Myeloid Leukaemia (CML), and has been central to the remarkable improvement in patient outcomes seen in this disease. As an ISO 17043 accredited international provider of External Quality Assessment (EQA)/Proficiency Testing (PT) in this area, UK NEQAS for Leucocyte Immunophenotyping (UKNEQAS LI) has a unique perspective on the evolving field of BCR-ABL1 testing in CML by having access to participants’ technical data.
Aims
To assess the impact of technical standardisation and the development of the International Scale (IS) on the accuracy of BCR-ABL1 testing, by reviewing our EQA trial data.
Methods
We analysed all trial data submitted to UKNEQAS LI that could be meaninfully compared between 2007 and 2015 (16 trial issues, 32 samples). In order to normalise the log normal EQA data, and allow the application of parametric statistics, a constant was applied to all participant data before log10 transformation. Robust statistics were used to assess variation, including the exclusion of outliers as per Algorithm A from ISO 5725-5. Whilst variation was calculated using log transformed data, where a meaningful data midpoint was required, medians were used because log transformed means (geometric means) are difficult to interpret and back transforming log transformed means is not recommended as it leads to underestimation.
Results
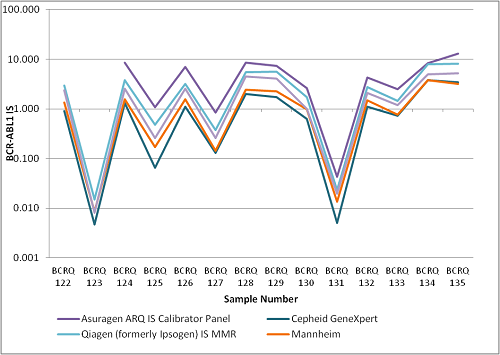
Comparison of participant results identified considerable variability at high and low levels of disease, including current and proposed therapeutically important levels. Despite rapid adoption of the IS by laboratories, and the fact that we have shown lower standard deviations in our IS data sets when compared to our unconverted data sets, suggestive of decreased variability, a statistically significant difference in variation between our unconverted and IS data sets could not be proven. We found that different methods of converting to the IS are producing consistently different median results within UKNEQAS LI IS data sets.
Conclusion
To allow better quantification of ‘Early’ and ‘Deep’ Molecular Response to fully realise the benefits of improved treatment regimens our data suggests that further refinement of RT-qPCR protocols, or perhaps a switch to new technologies such as droplet digital PCR, will be required. The finding that different methods of converting to the IS are producing consistently different median results suggests the need for a review of the process of converting to the IS.
Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Quality control, Real time PCR
Type: Eposter Presentation
Background
BCR-ABL1 measurement by RT-qPCR is integral to both the diagnosis and monitoring of Chronic Myeloid Leukaemia (CML), and has been central to the remarkable improvement in patient outcomes seen in this disease. As an ISO 17043 accredited international provider of External Quality Assessment (EQA)/Proficiency Testing (PT) in this area, UK NEQAS for Leucocyte Immunophenotyping (UKNEQAS LI) has a unique perspective on the evolving field of BCR-ABL1 testing in CML by having access to participants’ technical data.
Aims
To assess the impact of technical standardisation and the development of the International Scale (IS) on the accuracy of BCR-ABL1 testing, by reviewing our EQA trial data.
Methods
We analysed all trial data submitted to UKNEQAS LI that could be meaninfully compared between 2007 and 2015 (16 trial issues, 32 samples). In order to normalise the log normal EQA data, and allow the application of parametric statistics, a constant was applied to all participant data before log10 transformation. Robust statistics were used to assess variation, including the exclusion of outliers as per Algorithm A from ISO 5725-5. Whilst variation was calculated using log transformed data, where a meaningful data midpoint was required, medians were used because log transformed means (geometric means) are difficult to interpret and back transforming log transformed means is not recommended as it leads to underestimation.
Results
Comparison of participant results identified considerable variability at high and low levels of disease, including current and proposed therapeutically important levels. Despite rapid adoption of the IS by laboratories, and the fact that we have shown lower standard deviations in our IS data sets when compared to our unconverted data sets, suggestive of decreased variability, a statistically significant difference in variation between our unconverted and IS data sets could not be proven. We found that different methods of converting to the IS are producing consistently different median results within UKNEQAS LI IS data sets.
Conclusion
To allow better quantification of ‘Early’ and ‘Deep’ Molecular Response to fully realise the benefits of improved treatment regimens our data suggests that further refinement of RT-qPCR protocols, or perhaps a switch to new technologies such as droplet digital PCR, will be required. The finding that different methods of converting to the IS are producing consistently different median results suggests the need for a review of the process of converting to the IS.

Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Quality control, Real time PCR
Abstract: E1102
Type: Eposter Presentation
Background
BCR-ABL1 measurement by RT-qPCR is integral to both the diagnosis and monitoring of Chronic Myeloid Leukaemia (CML), and has been central to the remarkable improvement in patient outcomes seen in this disease. As an ISO 17043 accredited international provider of External Quality Assessment (EQA)/Proficiency Testing (PT) in this area, UK NEQAS for Leucocyte Immunophenotyping (UKNEQAS LI) has a unique perspective on the evolving field of BCR-ABL1 testing in CML by having access to participants’ technical data.
Aims
To assess the impact of technical standardisation and the development of the International Scale (IS) on the accuracy of BCR-ABL1 testing, by reviewing our EQA trial data.
Methods
We analysed all trial data submitted to UKNEQAS LI that could be meaninfully compared between 2007 and 2015 (16 trial issues, 32 samples). In order to normalise the log normal EQA data, and allow the application of parametric statistics, a constant was applied to all participant data before log10 transformation. Robust statistics were used to assess variation, including the exclusion of outliers as per Algorithm A from ISO 5725-5. Whilst variation was calculated using log transformed data, where a meaningful data midpoint was required, medians were used because log transformed means (geometric means) are difficult to interpret and back transforming log transformed means is not recommended as it leads to underestimation.
Results
Comparison of participant results identified considerable variability at high and low levels of disease, including current and proposed therapeutically important levels. Despite rapid adoption of the IS by laboratories, and the fact that we have shown lower standard deviations in our IS data sets when compared to our unconverted data sets, suggestive of decreased variability, a statistically significant difference in variation between our unconverted and IS data sets could not be proven. We found that different methods of converting to the IS are producing consistently different median results within UKNEQAS LI IS data sets.
Conclusion
To allow better quantification of ‘Early’ and ‘Deep’ Molecular Response to fully realise the benefits of improved treatment regimens our data suggests that further refinement of RT-qPCR protocols, or perhaps a switch to new technologies such as droplet digital PCR, will be required. The finding that different methods of converting to the IS are producing consistently different median results suggests the need for a review of the process of converting to the IS.

Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Quality control, Real time PCR
Type: Eposter Presentation
Background
BCR-ABL1 measurement by RT-qPCR is integral to both the diagnosis and monitoring of Chronic Myeloid Leukaemia (CML), and has been central to the remarkable improvement in patient outcomes seen in this disease. As an ISO 17043 accredited international provider of External Quality Assessment (EQA)/Proficiency Testing (PT) in this area, UK NEQAS for Leucocyte Immunophenotyping (UKNEQAS LI) has a unique perspective on the evolving field of BCR-ABL1 testing in CML by having access to participants’ technical data.
Aims
To assess the impact of technical standardisation and the development of the International Scale (IS) on the accuracy of BCR-ABL1 testing, by reviewing our EQA trial data.
Methods
We analysed all trial data submitted to UKNEQAS LI that could be meaninfully compared between 2007 and 2015 (16 trial issues, 32 samples). In order to normalise the log normal EQA data, and allow the application of parametric statistics, a constant was applied to all participant data before log10 transformation. Robust statistics were used to assess variation, including the exclusion of outliers as per Algorithm A from ISO 5725-5. Whilst variation was calculated using log transformed data, where a meaningful data midpoint was required, medians were used because log transformed means (geometric means) are difficult to interpret and back transforming log transformed means is not recommended as it leads to underestimation.
Results
Comparison of participant results identified considerable variability at high and low levels of disease, including current and proposed therapeutically important levels. Despite rapid adoption of the IS by laboratories, and the fact that we have shown lower standard deviations in our IS data sets when compared to our unconverted data sets, suggestive of decreased variability, a statistically significant difference in variation between our unconverted and IS data sets could not be proven. We found that different methods of converting to the IS are producing consistently different median results within UKNEQAS LI IS data sets.
Conclusion
To allow better quantification of ‘Early’ and ‘Deep’ Molecular Response to fully realise the benefits of improved treatment regimens our data suggests that further refinement of RT-qPCR protocols, or perhaps a switch to new technologies such as droplet digital PCR, will be required. The finding that different methods of converting to the IS are producing consistently different median results suggests the need for a review of the process of converting to the IS.

Session topic: E-poster
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Quality control, Real time PCR
{{ help_message }}
{{filter}}


