BCR-ABL FUSION TRANSCRIPT B2A2 IS ASSOCIATED WITH A HIGHER RISK OF NON-OPTIMAL EARLY RESPONSE IN CP-CML PATIENTS TREATED WITH STANDARD DOSE IMATINIB: A STUDY FROM GRUPPO TRIVENETO LMC
(Abstract release date: 05/19/16)
EHA Library. Binotto G. 06/09/16; 132648; E1099

Dr. Gianni Binotto
Contributions
Contributions
Abstract
Abstract: E1099
Type: Eposter Presentation
Background
The clinical significance of BCR-ABL transcript type in the tyrosine kinase inhibitors (TKI) era has recently been debated. However, few data concerning the impact of different BCR-ABL transcripts on early response dynamics in chronic phase-chronic myeloid leukemia (CP-CML) patients treated with standard dose imatinib (IM) have been reported.
Aims
To evaluate the probability of optimal response at early timepoints, according to different p210 BCR-ABL1 transcripts, in a real life population of CML-CP patients treated with standard dose front-line imatinib.
Methods
93 CP-CML patients treated with IM 400 mg daily, diagnosed from 2010 to 2015 were retrospectively analyzed. The type of BCR-ABL1 transcript was determined by multiplex RT-PCR at diagnosis. Molecular monitoring was performed with quantitative RT-PCR and results were expressed in International Scale (IS) values. Optimal, warning and failure categories were analyzed according to the 2013 ELN recommendations. Frequencies were compared by chi-squared test (χ2) or Fisher’s exact test. Progression-free survival (PFS) was calculated from the start of IM to ABP or death. Overall survival (OS) was calculated from the start of IM to death.
Results
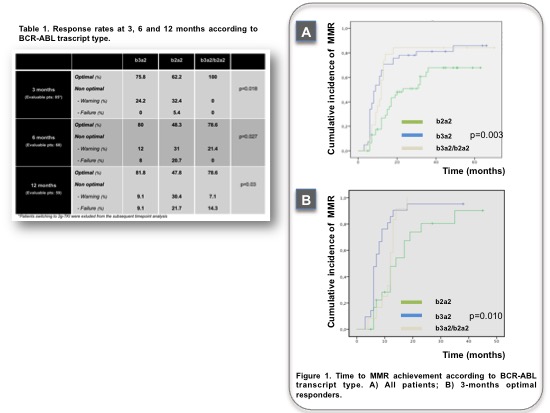
Patients with b3a2, b2a2, b3a2/b2a2 BCR-ABL transcript were 44.1%, 36.6% and 19.4%, respectively. The cohorts were comparable for age, sex and risk score (both Sokal and Eutos). Forty-three patients (46.2%) moved to second generation TKI (2GTKI), 24 (55.8%) due to primary or acquired cytogenetic or molecular resistance, 19 (44.2%) for intolerance. Rates of optimal vs non optimal response at 3 months were different between b3a2 vs b2a2 vs coexpressed transcript groups (75.8% vs 62.2% vs 100% respectively, p=0.018). Interestingly, all failures at 3 months were observed in the b2a2 group (Table 1). At 6 months, optimal responses were documented in 80% vs 48.3% vs 78.6% of b3a2, b2a2 and b3a2/b2a2 group, respectively (p=0.027); 60% of warnings and 75% of failures were associated to b2a2 transcript. At 12 months timepoint, major molecular response (MMR) rate was significantly different between transcripts (81.8% vs 47.8% vs 78.6% respectively, p=0.03). 52% of treatment changes within 12 months, either for resistance or intolerance, were observed in the b2a2 group. Differences in early response did not translate into inferior cumulative incidence of complete cytogenetic response (CCyR) (93.9% vs 82.1% vs 83.3% respectively, p=0.30) or different time to CCyR achievement. Patients with b3a2 and b3a2/b2a2 transcript had comparable rates of cumulative MMR (81.8% and 88.9%, respectively), superior to b2a2 patients (66.7%, p=0.12) with a significantly faster time to MMR achievement, either on IM treatment or with sequential IM+2GTKI approach (Figure 1A). Notably, among optimal responders at 3 months, MMR was obtained significantly later by patients with b2a2 transcript (Figure 1B). Long term outcomes (PFS, OS) were not affected by transcript type.
Conclusion
This retrospective analysis suggest a different kinetics of early optimal response achievement based on transcript type, with inferior rates in patients expressing b2a2. However, this difference did not translate into unfavourable clinical outcome. Longer time to MMR among optimal responders presenting with b2a2 could partially account for the negative impact of this transcript type on stable deep molecular response, previously observed by our group.
Session topic: E-poster
Keyword(s): BCR-ABL, Imatinib, Treatment
Type: Eposter Presentation
Background
The clinical significance of BCR-ABL transcript type in the tyrosine kinase inhibitors (TKI) era has recently been debated. However, few data concerning the impact of different BCR-ABL transcripts on early response dynamics in chronic phase-chronic myeloid leukemia (CP-CML) patients treated with standard dose imatinib (IM) have been reported.
Aims
To evaluate the probability of optimal response at early timepoints, according to different p210 BCR-ABL1 transcripts, in a real life population of CML-CP patients treated with standard dose front-line imatinib.
Methods
93 CP-CML patients treated with IM 400 mg daily, diagnosed from 2010 to 2015 were retrospectively analyzed. The type of BCR-ABL1 transcript was determined by multiplex RT-PCR at diagnosis. Molecular monitoring was performed with quantitative RT-PCR and results were expressed in International Scale (IS) values. Optimal, warning and failure categories were analyzed according to the 2013 ELN recommendations. Frequencies were compared by chi-squared test (χ2) or Fisher’s exact test. Progression-free survival (PFS) was calculated from the start of IM to ABP or death. Overall survival (OS) was calculated from the start of IM to death.
Results
Patients with b3a2, b2a2, b3a2/b2a2 BCR-ABL transcript were 44.1%, 36.6% and 19.4%, respectively. The cohorts were comparable for age, sex and risk score (both Sokal and Eutos). Forty-three patients (46.2%) moved to second generation TKI (2GTKI), 24 (55.8%) due to primary or acquired cytogenetic or molecular resistance, 19 (44.2%) for intolerance. Rates of optimal vs non optimal response at 3 months were different between b3a2 vs b2a2 vs coexpressed transcript groups (75.8% vs 62.2% vs 100% respectively, p=0.018). Interestingly, all failures at 3 months were observed in the b2a2 group (Table 1). At 6 months, optimal responses were documented in 80% vs 48.3% vs 78.6% of b3a2, b2a2 and b3a2/b2a2 group, respectively (p=0.027); 60% of warnings and 75% of failures were associated to b2a2 transcript. At 12 months timepoint, major molecular response (MMR) rate was significantly different between transcripts (81.8% vs 47.8% vs 78.6% respectively, p=0.03). 52% of treatment changes within 12 months, either for resistance or intolerance, were observed in the b2a2 group. Differences in early response did not translate into inferior cumulative incidence of complete cytogenetic response (CCyR) (93.9% vs 82.1% vs 83.3% respectively, p=0.30) or different time to CCyR achievement. Patients with b3a2 and b3a2/b2a2 transcript had comparable rates of cumulative MMR (81.8% and 88.9%, respectively), superior to b2a2 patients (66.7%, p=0.12) with a significantly faster time to MMR achievement, either on IM treatment or with sequential IM+2GTKI approach (Figure 1A). Notably, among optimal responders at 3 months, MMR was obtained significantly later by patients with b2a2 transcript (Figure 1B). Long term outcomes (PFS, OS) were not affected by transcript type.
Conclusion
This retrospective analysis suggest a different kinetics of early optimal response achievement based on transcript type, with inferior rates in patients expressing b2a2. However, this difference did not translate into unfavourable clinical outcome. Longer time to MMR among optimal responders presenting with b2a2 could partially account for the negative impact of this transcript type on stable deep molecular response, previously observed by our group.

Session topic: E-poster
Keyword(s): BCR-ABL, Imatinib, Treatment
Abstract: E1099
Type: Eposter Presentation
Background
The clinical significance of BCR-ABL transcript type in the tyrosine kinase inhibitors (TKI) era has recently been debated. However, few data concerning the impact of different BCR-ABL transcripts on early response dynamics in chronic phase-chronic myeloid leukemia (CP-CML) patients treated with standard dose imatinib (IM) have been reported.
Aims
To evaluate the probability of optimal response at early timepoints, according to different p210 BCR-ABL1 transcripts, in a real life population of CML-CP patients treated with standard dose front-line imatinib.
Methods
93 CP-CML patients treated with IM 400 mg daily, diagnosed from 2010 to 2015 were retrospectively analyzed. The type of BCR-ABL1 transcript was determined by multiplex RT-PCR at diagnosis. Molecular monitoring was performed with quantitative RT-PCR and results were expressed in International Scale (IS) values. Optimal, warning and failure categories were analyzed according to the 2013 ELN recommendations. Frequencies were compared by chi-squared test (χ2) or Fisher’s exact test. Progression-free survival (PFS) was calculated from the start of IM to ABP or death. Overall survival (OS) was calculated from the start of IM to death.
Results
Patients with b3a2, b2a2, b3a2/b2a2 BCR-ABL transcript were 44.1%, 36.6% and 19.4%, respectively. The cohorts were comparable for age, sex and risk score (both Sokal and Eutos). Forty-three patients (46.2%) moved to second generation TKI (2GTKI), 24 (55.8%) due to primary or acquired cytogenetic or molecular resistance, 19 (44.2%) for intolerance. Rates of optimal vs non optimal response at 3 months were different between b3a2 vs b2a2 vs coexpressed transcript groups (75.8% vs 62.2% vs 100% respectively, p=0.018). Interestingly, all failures at 3 months were observed in the b2a2 group (Table 1). At 6 months, optimal responses were documented in 80% vs 48.3% vs 78.6% of b3a2, b2a2 and b3a2/b2a2 group, respectively (p=0.027); 60% of warnings and 75% of failures were associated to b2a2 transcript. At 12 months timepoint, major molecular response (MMR) rate was significantly different between transcripts (81.8% vs 47.8% vs 78.6% respectively, p=0.03). 52% of treatment changes within 12 months, either for resistance or intolerance, were observed in the b2a2 group. Differences in early response did not translate into inferior cumulative incidence of complete cytogenetic response (CCyR) (93.9% vs 82.1% vs 83.3% respectively, p=0.30) or different time to CCyR achievement. Patients with b3a2 and b3a2/b2a2 transcript had comparable rates of cumulative MMR (81.8% and 88.9%, respectively), superior to b2a2 patients (66.7%, p=0.12) with a significantly faster time to MMR achievement, either on IM treatment or with sequential IM+2GTKI approach (Figure 1A). Notably, among optimal responders at 3 months, MMR was obtained significantly later by patients with b2a2 transcript (Figure 1B). Long term outcomes (PFS, OS) were not affected by transcript type.
Conclusion
This retrospective analysis suggest a different kinetics of early optimal response achievement based on transcript type, with inferior rates in patients expressing b2a2. However, this difference did not translate into unfavourable clinical outcome. Longer time to MMR among optimal responders presenting with b2a2 could partially account for the negative impact of this transcript type on stable deep molecular response, previously observed by our group.

Session topic: E-poster
Keyword(s): BCR-ABL, Imatinib, Treatment
Type: Eposter Presentation
Background
The clinical significance of BCR-ABL transcript type in the tyrosine kinase inhibitors (TKI) era has recently been debated. However, few data concerning the impact of different BCR-ABL transcripts on early response dynamics in chronic phase-chronic myeloid leukemia (CP-CML) patients treated with standard dose imatinib (IM) have been reported.
Aims
To evaluate the probability of optimal response at early timepoints, according to different p210 BCR-ABL1 transcripts, in a real life population of CML-CP patients treated with standard dose front-line imatinib.
Methods
93 CP-CML patients treated with IM 400 mg daily, diagnosed from 2010 to 2015 were retrospectively analyzed. The type of BCR-ABL1 transcript was determined by multiplex RT-PCR at diagnosis. Molecular monitoring was performed with quantitative RT-PCR and results were expressed in International Scale (IS) values. Optimal, warning and failure categories were analyzed according to the 2013 ELN recommendations. Frequencies were compared by chi-squared test (χ2) or Fisher’s exact test. Progression-free survival (PFS) was calculated from the start of IM to ABP or death. Overall survival (OS) was calculated from the start of IM to death.
Results
Patients with b3a2, b2a2, b3a2/b2a2 BCR-ABL transcript were 44.1%, 36.6% and 19.4%, respectively. The cohorts were comparable for age, sex and risk score (both Sokal and Eutos). Forty-three patients (46.2%) moved to second generation TKI (2GTKI), 24 (55.8%) due to primary or acquired cytogenetic or molecular resistance, 19 (44.2%) for intolerance. Rates of optimal vs non optimal response at 3 months were different between b3a2 vs b2a2 vs coexpressed transcript groups (75.8% vs 62.2% vs 100% respectively, p=0.018). Interestingly, all failures at 3 months were observed in the b2a2 group (Table 1). At 6 months, optimal responses were documented in 80% vs 48.3% vs 78.6% of b3a2, b2a2 and b3a2/b2a2 group, respectively (p=0.027); 60% of warnings and 75% of failures were associated to b2a2 transcript. At 12 months timepoint, major molecular response (MMR) rate was significantly different between transcripts (81.8% vs 47.8% vs 78.6% respectively, p=0.03). 52% of treatment changes within 12 months, either for resistance or intolerance, were observed in the b2a2 group. Differences in early response did not translate into inferior cumulative incidence of complete cytogenetic response (CCyR) (93.9% vs 82.1% vs 83.3% respectively, p=0.30) or different time to CCyR achievement. Patients with b3a2 and b3a2/b2a2 transcript had comparable rates of cumulative MMR (81.8% and 88.9%, respectively), superior to b2a2 patients (66.7%, p=0.12) with a significantly faster time to MMR achievement, either on IM treatment or with sequential IM+2GTKI approach (Figure 1A). Notably, among optimal responders at 3 months, MMR was obtained significantly later by patients with b2a2 transcript (Figure 1B). Long term outcomes (PFS, OS) were not affected by transcript type.
Conclusion
This retrospective analysis suggest a different kinetics of early optimal response achievement based on transcript type, with inferior rates in patients expressing b2a2. However, this difference did not translate into unfavourable clinical outcome. Longer time to MMR among optimal responders presenting with b2a2 could partially account for the negative impact of this transcript type on stable deep molecular response, previously observed by our group.

Session topic: E-poster
Keyword(s): BCR-ABL, Imatinib, Treatment
{{ help_message }}
{{filter}}


