PERFORMANCE STATUS AND COMORBIDITY AFFECT SURVIVAL IN ELDERLY PATIENTS WITH CLL AFTER LOW DOSE ALEMTUZUMAB COMBINED WITH CHEMOTHERAPY: RESULTS FROM THE HOVON68 TRIAL.
(Abstract release date: 05/19/16)
EHA Library. Vojdeman F. 06/09/16; 132621; E1072

Dr. Fie Vojdeman
Contributions
Contributions
Abstract
Abstract: E1072
Type: Eposter Presentation
Background
In CLL, chemo-immunotherapy is the standard of care. Until recently fludarabine and cyclophosphamide (FC) has been considered the chemotherapy of choice in fit patients irrespective of age. However, ageing results in a decline in organ function and immune competence resulting in higher susceptibility to the toxicity of chemotherapy, while FC+Rituximab resulted in more infections when compared to Bendamustine+Rituximab, also in the elderly (Eichhorst et al. 2014). Furthermore, in the HOVON68 CLL trial, patients above 65 years of age had no survival benefit from the addition of low-dose alemtuzumab to FC in contrast to younger patients (Geisler et al. 2014).
Aims
Here we address the issue of why elderly patients with CLL did not benefit from the addition of low-dose alemtuzumab to FC, using a 5 year update of the HOVON68 trial.
Methods
272 patients from the HOVON68 trial were included in this study. All data were collected from the HOVON database, updated as of February 2015, to reach a median follow-up period of 61 months. Baseline characteristics, causes of death, comorbidities, organ related adverse events, infections, and lymphocyte counts during treatment and the follow-up period were extracted as variables. The statistical analyses included survival- and competing risk analysis using univariate and multivariate cox regression performed in STATA version 13.1. P-values were two-sided and considered as statistically significant if below 0.05.
Results
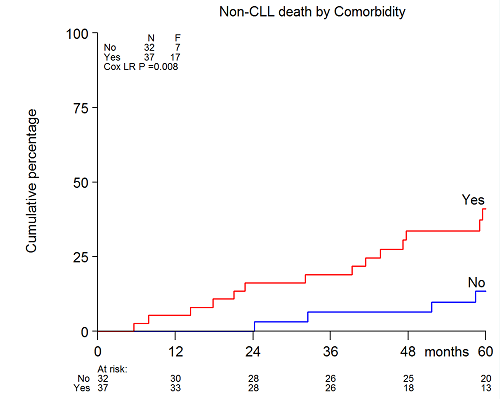
The addition of alemtuzumab to FC (FCA) improved overall survival from a median of 77 months to median not yet reached (log rank P=0.049) in the 5 year update of the HOVON68 trial. In addition, we confirmed that elderly patients aged 65 to 75 of age had no improvement in OS (p=0.38). Overall 40% (110/272) of patients died in the follow-up period. Excluding deaths related to allogeneic transplant (n=10), 44% (44/100) died from CLL-related causes. The elderly patients who received FCA had close to a significantly higher non-CLL related death rate compared to all others (p=0.05). Infections accounted for almost half (46.4%) of all non-CLL related causes of death. However, among FCA treated patients, non-CLL related deaths in the elderly were primarily due to secondary cancer or cardiovascular disease (10/14, 71%). At trial entry, most of the comorbidity in the elderly was cardiovascular (23/37, 62%) including hypertension, atrial fibrillation, history of transient cerebral ischemia, or ischemic heart disease. The comorbidities were balanced between the treatment arms.In the elderly, performance status (PS)>0, del(17p), β2microglobulin≥3.5mg/dL, opportunistic infection, and comorbidity>0 (Figure 1) were significantly associated with non-CLL related mortality by univariate Cox regression using competing risk analysis. By multivariate analysis of factors found to be significant on univariate analysis, only PS>0 (HR 5.25 [1.88-14.63], p=0.002), del(17p) (HR 4.47 [1.35-14.81], p=0.014), and comorbidity (HR 5.4 [1.66-15.33], p=0.004) remained independently significant. No interactions were found between the three significant variables with treatment arm, or between PS and comorbidity.
Conclusion
FCA induced more non-CLL and non-infection related deaths in elderly patients compared to younger patients with biological high risk CLL in the HOVON68 trial. Both PS and comorbidity had a significant impact on non-CLL related mortality in the elderly after treatment with FC or FCA.
Session topic: E-poster
Keyword(s): B cell chronic lymphocytic leukemia, CAMPATH-1H, Clinical trial, Comorbidities
Type: Eposter Presentation
Background
In CLL, chemo-immunotherapy is the standard of care. Until recently fludarabine and cyclophosphamide (FC) has been considered the chemotherapy of choice in fit patients irrespective of age. However, ageing results in a decline in organ function and immune competence resulting in higher susceptibility to the toxicity of chemotherapy, while FC+Rituximab resulted in more infections when compared to Bendamustine+Rituximab, also in the elderly (Eichhorst et al. 2014). Furthermore, in the HOVON68 CLL trial, patients above 65 years of age had no survival benefit from the addition of low-dose alemtuzumab to FC in contrast to younger patients (Geisler et al. 2014).
Aims
Here we address the issue of why elderly patients with CLL did not benefit from the addition of low-dose alemtuzumab to FC, using a 5 year update of the HOVON68 trial.
Methods
272 patients from the HOVON68 trial were included in this study. All data were collected from the HOVON database, updated as of February 2015, to reach a median follow-up period of 61 months. Baseline characteristics, causes of death, comorbidities, organ related adverse events, infections, and lymphocyte counts during treatment and the follow-up period were extracted as variables. The statistical analyses included survival- and competing risk analysis using univariate and multivariate cox regression performed in STATA version 13.1. P-values were two-sided and considered as statistically significant if below 0.05.
Results
The addition of alemtuzumab to FC (FCA) improved overall survival from a median of 77 months to median not yet reached (log rank P=0.049) in the 5 year update of the HOVON68 trial. In addition, we confirmed that elderly patients aged 65 to 75 of age had no improvement in OS (p=0.38). Overall 40% (110/272) of patients died in the follow-up period. Excluding deaths related to allogeneic transplant (n=10), 44% (44/100) died from CLL-related causes. The elderly patients who received FCA had close to a significantly higher non-CLL related death rate compared to all others (p=0.05). Infections accounted for almost half (46.4%) of all non-CLL related causes of death. However, among FCA treated patients, non-CLL related deaths in the elderly were primarily due to secondary cancer or cardiovascular disease (10/14, 71%). At trial entry, most of the comorbidity in the elderly was cardiovascular (23/37, 62%) including hypertension, atrial fibrillation, history of transient cerebral ischemia, or ischemic heart disease. The comorbidities were balanced between the treatment arms.In the elderly, performance status (PS)>0, del(17p), β2microglobulin≥3.5mg/dL, opportunistic infection, and comorbidity>0 (Figure 1) were significantly associated with non-CLL related mortality by univariate Cox regression using competing risk analysis. By multivariate analysis of factors found to be significant on univariate analysis, only PS>0 (HR 5.25 [1.88-14.63], p=0.002), del(17p) (HR 4.47 [1.35-14.81], p=0.014), and comorbidity (HR 5.4 [1.66-15.33], p=0.004) remained independently significant. No interactions were found between the three significant variables with treatment arm, or between PS and comorbidity.
Conclusion
FCA induced more non-CLL and non-infection related deaths in elderly patients compared to younger patients with biological high risk CLL in the HOVON68 trial. Both PS and comorbidity had a significant impact on non-CLL related mortality in the elderly after treatment with FC or FCA.

Session topic: E-poster
Keyword(s): B cell chronic lymphocytic leukemia, CAMPATH-1H, Clinical trial, Comorbidities
Abstract: E1072
Type: Eposter Presentation
Background
In CLL, chemo-immunotherapy is the standard of care. Until recently fludarabine and cyclophosphamide (FC) has been considered the chemotherapy of choice in fit patients irrespective of age. However, ageing results in a decline in organ function and immune competence resulting in higher susceptibility to the toxicity of chemotherapy, while FC+Rituximab resulted in more infections when compared to Bendamustine+Rituximab, also in the elderly (Eichhorst et al. 2014). Furthermore, in the HOVON68 CLL trial, patients above 65 years of age had no survival benefit from the addition of low-dose alemtuzumab to FC in contrast to younger patients (Geisler et al. 2014).
Aims
Here we address the issue of why elderly patients with CLL did not benefit from the addition of low-dose alemtuzumab to FC, using a 5 year update of the HOVON68 trial.
Methods
272 patients from the HOVON68 trial were included in this study. All data were collected from the HOVON database, updated as of February 2015, to reach a median follow-up period of 61 months. Baseline characteristics, causes of death, comorbidities, organ related adverse events, infections, and lymphocyte counts during treatment and the follow-up period were extracted as variables. The statistical analyses included survival- and competing risk analysis using univariate and multivariate cox regression performed in STATA version 13.1. P-values were two-sided and considered as statistically significant if below 0.05.
Results
The addition of alemtuzumab to FC (FCA) improved overall survival from a median of 77 months to median not yet reached (log rank P=0.049) in the 5 year update of the HOVON68 trial. In addition, we confirmed that elderly patients aged 65 to 75 of age had no improvement in OS (p=0.38). Overall 40% (110/272) of patients died in the follow-up period. Excluding deaths related to allogeneic transplant (n=10), 44% (44/100) died from CLL-related causes. The elderly patients who received FCA had close to a significantly higher non-CLL related death rate compared to all others (p=0.05). Infections accounted for almost half (46.4%) of all non-CLL related causes of death. However, among FCA treated patients, non-CLL related deaths in the elderly were primarily due to secondary cancer or cardiovascular disease (10/14, 71%). At trial entry, most of the comorbidity in the elderly was cardiovascular (23/37, 62%) including hypertension, atrial fibrillation, history of transient cerebral ischemia, or ischemic heart disease. The comorbidities were balanced between the treatment arms.In the elderly, performance status (PS)>0, del(17p), β2microglobulin≥3.5mg/dL, opportunistic infection, and comorbidity>0 (Figure 1) were significantly associated with non-CLL related mortality by univariate Cox regression using competing risk analysis. By multivariate analysis of factors found to be significant on univariate analysis, only PS>0 (HR 5.25 [1.88-14.63], p=0.002), del(17p) (HR 4.47 [1.35-14.81], p=0.014), and comorbidity (HR 5.4 [1.66-15.33], p=0.004) remained independently significant. No interactions were found between the three significant variables with treatment arm, or between PS and comorbidity.
Conclusion
FCA induced more non-CLL and non-infection related deaths in elderly patients compared to younger patients with biological high risk CLL in the HOVON68 trial. Both PS and comorbidity had a significant impact on non-CLL related mortality in the elderly after treatment with FC or FCA.

Session topic: E-poster
Keyword(s): B cell chronic lymphocytic leukemia, CAMPATH-1H, Clinical trial, Comorbidities
Type: Eposter Presentation
Background
In CLL, chemo-immunotherapy is the standard of care. Until recently fludarabine and cyclophosphamide (FC) has been considered the chemotherapy of choice in fit patients irrespective of age. However, ageing results in a decline in organ function and immune competence resulting in higher susceptibility to the toxicity of chemotherapy, while FC+Rituximab resulted in more infections when compared to Bendamustine+Rituximab, also in the elderly (Eichhorst et al. 2014). Furthermore, in the HOVON68 CLL trial, patients above 65 years of age had no survival benefit from the addition of low-dose alemtuzumab to FC in contrast to younger patients (Geisler et al. 2014).
Aims
Here we address the issue of why elderly patients with CLL did not benefit from the addition of low-dose alemtuzumab to FC, using a 5 year update of the HOVON68 trial.
Methods
272 patients from the HOVON68 trial were included in this study. All data were collected from the HOVON database, updated as of February 2015, to reach a median follow-up period of 61 months. Baseline characteristics, causes of death, comorbidities, organ related adverse events, infections, and lymphocyte counts during treatment and the follow-up period were extracted as variables. The statistical analyses included survival- and competing risk analysis using univariate and multivariate cox regression performed in STATA version 13.1. P-values were two-sided and considered as statistically significant if below 0.05.
Results
The addition of alemtuzumab to FC (FCA) improved overall survival from a median of 77 months to median not yet reached (log rank P=0.049) in the 5 year update of the HOVON68 trial. In addition, we confirmed that elderly patients aged 65 to 75 of age had no improvement in OS (p=0.38). Overall 40% (110/272) of patients died in the follow-up period. Excluding deaths related to allogeneic transplant (n=10), 44% (44/100) died from CLL-related causes. The elderly patients who received FCA had close to a significantly higher non-CLL related death rate compared to all others (p=0.05). Infections accounted for almost half (46.4%) of all non-CLL related causes of death. However, among FCA treated patients, non-CLL related deaths in the elderly were primarily due to secondary cancer or cardiovascular disease (10/14, 71%). At trial entry, most of the comorbidity in the elderly was cardiovascular (23/37, 62%) including hypertension, atrial fibrillation, history of transient cerebral ischemia, or ischemic heart disease. The comorbidities were balanced between the treatment arms.In the elderly, performance status (PS)>0, del(17p), β2microglobulin≥3.5mg/dL, opportunistic infection, and comorbidity>0 (Figure 1) were significantly associated with non-CLL related mortality by univariate Cox regression using competing risk analysis. By multivariate analysis of factors found to be significant on univariate analysis, only PS>0 (HR 5.25 [1.88-14.63], p=0.002), del(17p) (HR 4.47 [1.35-14.81], p=0.014), and comorbidity (HR 5.4 [1.66-15.33], p=0.004) remained independently significant. No interactions were found between the three significant variables with treatment arm, or between PS and comorbidity.
Conclusion
FCA induced more non-CLL and non-infection related deaths in elderly patients compared to younger patients with biological high risk CLL in the HOVON68 trial. Both PS and comorbidity had a significant impact on non-CLL related mortality in the elderly after treatment with FC or FCA.

Session topic: E-poster
Keyword(s): B cell chronic lymphocytic leukemia, CAMPATH-1H, Clinical trial, Comorbidities
{{ help_message }}
{{filter}}


