CD49D EXPRESSION IS ASSOCIATED WITH DEVELOPMENT OF LYMPHADENOPATHY IN PATIENTS WITH MONOCLONAL B-CELL LYMPHOCYTOSIS (MBL) AND CHRONIC LYMPHOCYTIC LEUKEMIA (CLL)
(Abstract release date: 05/19/16)
EHA Library. Strati P. 06/09/16; 132608; E1059

Dr. Paolo Strati
Contributions
Contributions
Abstract
Abstract: E1059
Type: Eposter Presentation
Background
CLL clinical presentation is very heterogeneous, including lymphadenopathy, organomegaly, and cytopenias. The biologic factors which determine these differences in clinical manifestation are poorly understood. CD49d is a surface integrin expressed on CLL lymphocytes that facilitates interactions between CLL B-cells and stromal cells in the microenvironment. Although case series suggest high expression of CD49d in CLL patients may be associated with presentation with lymphadenopathy, these series are small and nearly all the information to date is cross-sectional.
Aims
We used the Mayo Clinic CLL database to prospectively evaluate the association between CD49d expression and subsequent development of lymphadenopathy in a cohort of patients with newly diagnosed MBL and CLL Rai stage 0.
Methods
All patients seen at our center between 08/2001 and 12/2015 and who had pre-treatment CD49d testing available within 1 year of the date of diagnosis were included in the study. CD49d was considered positive if >30% of cells expressed CD49d (JCO 32:897). Time to development of lymphadenopathy was defined as time from diagnosis to appearance of palpable lymph nodes; in the absence of development of lymphadenopathy, patients were censored at date of first therapy or last follow-up. The Kaplan-Meier plots display time to development of lymphadenopathy.
Results
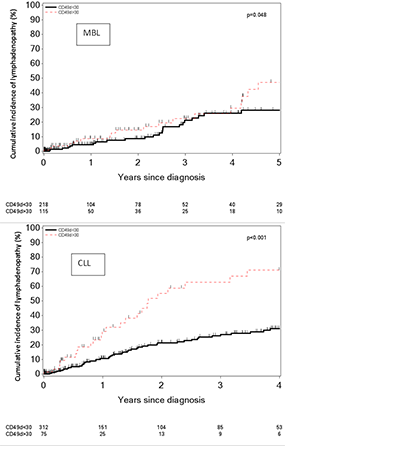
The study included 720 individuals, 333 with MBL and 387 with Rai stage 0 CLL. Among these patients, CD49d was positive in 34.5% of those with MBL and in 19.4% of patients with CLL Rai stage 0 (p<0.001). The median % of clonal B-cells expressing CD49d at diagnosis was 12% (interquartile range [IQR] 5%>59%) in individuals with MBL and 3.7% (IQR, 2%>14%) in patients with Rai stage 0 (p<0.001). Fourty-four (15%) patients with MBL and 82 (21%) patients with CLL Rai 0 subsequently developed palpable lymphadenopathy during the course of follow-up (median follow-up= 3.4 years). CD49d positivity at baseline was also associated with shorter time to development of lymphadenopathy both in patients with MBL (p=0.048) and CLL Rai 0 (p<0.001; Figure).
Conclusion
CD49d expression identifies a cohort of MBL and Rai 0 CLL patients whose clinical course may be dominated by development of lymphadenopathy as opposed to bone marrow infiltration and cytopenias.
Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin
Type: Eposter Presentation
Background
CLL clinical presentation is very heterogeneous, including lymphadenopathy, organomegaly, and cytopenias. The biologic factors which determine these differences in clinical manifestation are poorly understood. CD49d is a surface integrin expressed on CLL lymphocytes that facilitates interactions between CLL B-cells and stromal cells in the microenvironment. Although case series suggest high expression of CD49d in CLL patients may be associated with presentation with lymphadenopathy, these series are small and nearly all the information to date is cross-sectional.
Aims
We used the Mayo Clinic CLL database to prospectively evaluate the association between CD49d expression and subsequent development of lymphadenopathy in a cohort of patients with newly diagnosed MBL and CLL Rai stage 0.
Methods
All patients seen at our center between 08/2001 and 12/2015 and who had pre-treatment CD49d testing available within 1 year of the date of diagnosis were included in the study. CD49d was considered positive if >30% of cells expressed CD49d (JCO 32:897). Time to development of lymphadenopathy was defined as time from diagnosis to appearance of palpable lymph nodes; in the absence of development of lymphadenopathy, patients were censored at date of first therapy or last follow-up. The Kaplan-Meier plots display time to development of lymphadenopathy.
Results
The study included 720 individuals, 333 with MBL and 387 with Rai stage 0 CLL. Among these patients, CD49d was positive in 34.5% of those with MBL and in 19.4% of patients with CLL Rai stage 0 (p<0.001). The median % of clonal B-cells expressing CD49d at diagnosis was 12% (interquartile range [IQR] 5%>59%) in individuals with MBL and 3.7% (IQR, 2%>14%) in patients with Rai stage 0 (p<0.001). Fourty-four (15%) patients with MBL and 82 (21%) patients with CLL Rai 0 subsequently developed palpable lymphadenopathy during the course of follow-up (median follow-up= 3.4 years). CD49d positivity at baseline was also associated with shorter time to development of lymphadenopathy both in patients with MBL (p=0.048) and CLL Rai 0 (p<0.001; Figure).
Conclusion
CD49d expression identifies a cohort of MBL and Rai 0 CLL patients whose clinical course may be dominated by development of lymphadenopathy as opposed to bone marrow infiltration and cytopenias.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin
Abstract: E1059
Type: Eposter Presentation
Background
CLL clinical presentation is very heterogeneous, including lymphadenopathy, organomegaly, and cytopenias. The biologic factors which determine these differences in clinical manifestation are poorly understood. CD49d is a surface integrin expressed on CLL lymphocytes that facilitates interactions between CLL B-cells and stromal cells in the microenvironment. Although case series suggest high expression of CD49d in CLL patients may be associated with presentation with lymphadenopathy, these series are small and nearly all the information to date is cross-sectional.
Aims
We used the Mayo Clinic CLL database to prospectively evaluate the association between CD49d expression and subsequent development of lymphadenopathy in a cohort of patients with newly diagnosed MBL and CLL Rai stage 0.
Methods
All patients seen at our center between 08/2001 and 12/2015 and who had pre-treatment CD49d testing available within 1 year of the date of diagnosis were included in the study. CD49d was considered positive if >30% of cells expressed CD49d (JCO 32:897). Time to development of lymphadenopathy was defined as time from diagnosis to appearance of palpable lymph nodes; in the absence of development of lymphadenopathy, patients were censored at date of first therapy or last follow-up. The Kaplan-Meier plots display time to development of lymphadenopathy.
Results
The study included 720 individuals, 333 with MBL and 387 with Rai stage 0 CLL. Among these patients, CD49d was positive in 34.5% of those with MBL and in 19.4% of patients with CLL Rai stage 0 (p<0.001). The median % of clonal B-cells expressing CD49d at diagnosis was 12% (interquartile range [IQR] 5%>59%) in individuals with MBL and 3.7% (IQR, 2%>14%) in patients with Rai stage 0 (p<0.001). Fourty-four (15%) patients with MBL and 82 (21%) patients with CLL Rai 0 subsequently developed palpable lymphadenopathy during the course of follow-up (median follow-up= 3.4 years). CD49d positivity at baseline was also associated with shorter time to development of lymphadenopathy both in patients with MBL (p=0.048) and CLL Rai 0 (p<0.001; Figure).
Conclusion
CD49d expression identifies a cohort of MBL and Rai 0 CLL patients whose clinical course may be dominated by development of lymphadenopathy as opposed to bone marrow infiltration and cytopenias.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin
Type: Eposter Presentation
Background
CLL clinical presentation is very heterogeneous, including lymphadenopathy, organomegaly, and cytopenias. The biologic factors which determine these differences in clinical manifestation are poorly understood. CD49d is a surface integrin expressed on CLL lymphocytes that facilitates interactions between CLL B-cells and stromal cells in the microenvironment. Although case series suggest high expression of CD49d in CLL patients may be associated with presentation with lymphadenopathy, these series are small and nearly all the information to date is cross-sectional.
Aims
We used the Mayo Clinic CLL database to prospectively evaluate the association between CD49d expression and subsequent development of lymphadenopathy in a cohort of patients with newly diagnosed MBL and CLL Rai stage 0.
Methods
All patients seen at our center between 08/2001 and 12/2015 and who had pre-treatment CD49d testing available within 1 year of the date of diagnosis were included in the study. CD49d was considered positive if >30% of cells expressed CD49d (JCO 32:897). Time to development of lymphadenopathy was defined as time from diagnosis to appearance of palpable lymph nodes; in the absence of development of lymphadenopathy, patients were censored at date of first therapy or last follow-up. The Kaplan-Meier plots display time to development of lymphadenopathy.
Results
The study included 720 individuals, 333 with MBL and 387 with Rai stage 0 CLL. Among these patients, CD49d was positive in 34.5% of those with MBL and in 19.4% of patients with CLL Rai stage 0 (p<0.001). The median % of clonal B-cells expressing CD49d at diagnosis was 12% (interquartile range [IQR] 5%>59%) in individuals with MBL and 3.7% (IQR, 2%>14%) in patients with Rai stage 0 (p<0.001). Fourty-four (15%) patients with MBL and 82 (21%) patients with CLL Rai 0 subsequently developed palpable lymphadenopathy during the course of follow-up (median follow-up= 3.4 years). CD49d positivity at baseline was also associated with shorter time to development of lymphadenopathy both in patients with MBL (p=0.048) and CLL Rai 0 (p<0.001; Figure).
Conclusion
CD49d expression identifies a cohort of MBL and Rai 0 CLL patients whose clinical course may be dominated by development of lymphadenopathy as opposed to bone marrow infiltration and cytopenias.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin
{{ help_message }}
{{filter}}


