IDELALISIB IN COMBINATION WITH BENDAMUSTINE/RITUXIMAB IMPROVES OVERALL SURVIVAL IN PATIENTS WITH RELAPSED/REFRACTORY CLL: INTERIM RESULTS OF A PHASE 3 RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED STUDY
(Abstract release date: 05/19/16)
EHA Library. Hillmen P. 06/09/16; 132601; E1052

Prof. Peter Hillmen
Contributions
Contributions
Abstract
Abstract: E1052
Type: Eposter Presentation
Background
Patients (pts) with relapsed/refractory CLL and adverse prognostic features (e.g., del17p/TP53mut) respond poorly to standard treatment. Here we present results of a pre-specified subgroup analysis of OS and ORR from an ongoing randomized double-blind placebo-controlled study (ClinicalTrials.gov ID: NCT01569295).
Aims
To demonstrate an improvement in OS and ORR in pts on idelalisib (IDELA) in combination with bendamustine/rituximab vs bendamustine/rituximab alone in pts with relapsed/refractory CLL and in a pre-specified pt population with adverse risk features.
Methods
Between June 2012 and August 2014, 416 pts with relapsed/refractory CLL from 180 centers in North America, Australia, New Zealand, and Europe were enrolled in this ongoing study. After informed consent, eligible pts were randomized 1:1 to idelalisib 150 mg BID (207 pts) or matching placebo (209 pts). All pts received bendamustine 70 mg/m2 on days 1 & 2 and rituximab 375 mg/m2 day 1 of cycle 1 and 500 mg/m2 D1 cycles 2 to 6. A cycle of the regimen was Q28 days. Study treatment continued until disease progression, death, intolerable toxicity, or withdrawn consent. Stratification performed by an independent laboratory was by del17p, TP53 mutations, or mutated IGHV, and clinically by refractory (progression <6 months from completion of prior therapy) or relapsed (progression at least 6 months from completion of prior therapy) disease. Median follow-up at the time of this report was 12 months. The primary endpoint of PFS defined by standard criteria was adjudicated by an IRC. Overall survival and ORR were secondary endpoints. Crossover was not permitted at the time of CLL disease progression.
Results
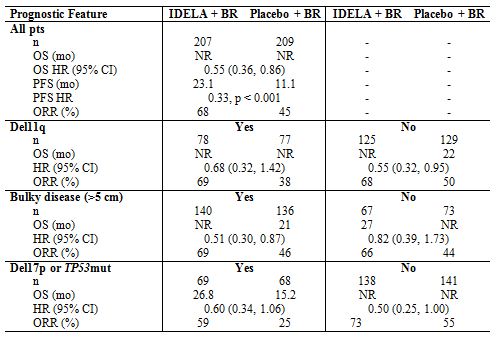
Median overall survival was not reached in either arm; there was a statistically significant improvement in overall survival, after the pre-specified multiplicity adjustment, in the idelalisib arm (HR = 0.55; 95% CI: 0.36, 0.86; P = 0.008 [stratified]). As of 15 June 2015, there were 34 deaths in the idelalisib arm and 51 deaths in the placebo arm. An improvement in ORR was observed on the idelalisib vs placebo arm in all adverse-risk categories evaluated (Table).
Conclusion
Idelalisib in combination with bendamustine and rituximab is superior to bendamustine and rituximab alone, reducing the risk of death and disease progression, and increasing progression-free and overall survival. Overall response rate was also increased on the idelalisib arm. These results were consistent across pts with adverse-risk features. The safety profile was consistent with prior reported studies. This trial provides further evidence for the improved outcomes for idelalisib-based therapy in pts with relapsed/refractory CLL. This regimen represents an important new treatment option for the management of relapsed/refractory CLL, further establishing the role of idelalisib in this setting.
Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Phase III, PI3K
Type: Eposter Presentation
Background
Patients (pts) with relapsed/refractory CLL and adverse prognostic features (e.g., del17p/TP53mut) respond poorly to standard treatment. Here we present results of a pre-specified subgroup analysis of OS and ORR from an ongoing randomized double-blind placebo-controlled study (ClinicalTrials.gov ID: NCT01569295).
Aims
To demonstrate an improvement in OS and ORR in pts on idelalisib (IDELA) in combination with bendamustine/rituximab vs bendamustine/rituximab alone in pts with relapsed/refractory CLL and in a pre-specified pt population with adverse risk features.
Methods
Between June 2012 and August 2014, 416 pts with relapsed/refractory CLL from 180 centers in North America, Australia, New Zealand, and Europe were enrolled in this ongoing study. After informed consent, eligible pts were randomized 1:1 to idelalisib 150 mg BID (207 pts) or matching placebo (209 pts). All pts received bendamustine 70 mg/m2 on days 1 & 2 and rituximab 375 mg/m2 day 1 of cycle 1 and 500 mg/m2 D1 cycles 2 to 6. A cycle of the regimen was Q28 days. Study treatment continued until disease progression, death, intolerable toxicity, or withdrawn consent. Stratification performed by an independent laboratory was by del17p, TP53 mutations, or mutated IGHV, and clinically by refractory (progression <6 months from completion of prior therapy) or relapsed (progression at least 6 months from completion of prior therapy) disease. Median follow-up at the time of this report was 12 months. The primary endpoint of PFS defined by standard criteria was adjudicated by an IRC. Overall survival and ORR were secondary endpoints. Crossover was not permitted at the time of CLL disease progression.
Results
Median overall survival was not reached in either arm; there was a statistically significant improvement in overall survival, after the pre-specified multiplicity adjustment, in the idelalisib arm (HR = 0.55; 95% CI: 0.36, 0.86; P = 0.008 [stratified]). As of 15 June 2015, there were 34 deaths in the idelalisib arm and 51 deaths in the placebo arm. An improvement in ORR was observed on the idelalisib vs placebo arm in all adverse-risk categories evaluated (Table).
Conclusion
Idelalisib in combination with bendamustine and rituximab is superior to bendamustine and rituximab alone, reducing the risk of death and disease progression, and increasing progression-free and overall survival. Overall response rate was also increased on the idelalisib arm. These results were consistent across pts with adverse-risk features. The safety profile was consistent with prior reported studies. This trial provides further evidence for the improved outcomes for idelalisib-based therapy in pts with relapsed/refractory CLL. This regimen represents an important new treatment option for the management of relapsed/refractory CLL, further establishing the role of idelalisib in this setting.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Phase III, PI3K
Abstract: E1052
Type: Eposter Presentation
Background
Patients (pts) with relapsed/refractory CLL and adverse prognostic features (e.g., del17p/TP53mut) respond poorly to standard treatment. Here we present results of a pre-specified subgroup analysis of OS and ORR from an ongoing randomized double-blind placebo-controlled study (ClinicalTrials.gov ID: NCT01569295).
Aims
To demonstrate an improvement in OS and ORR in pts on idelalisib (IDELA) in combination with bendamustine/rituximab vs bendamustine/rituximab alone in pts with relapsed/refractory CLL and in a pre-specified pt population with adverse risk features.
Methods
Between June 2012 and August 2014, 416 pts with relapsed/refractory CLL from 180 centers in North America, Australia, New Zealand, and Europe were enrolled in this ongoing study. After informed consent, eligible pts were randomized 1:1 to idelalisib 150 mg BID (207 pts) or matching placebo (209 pts). All pts received bendamustine 70 mg/m2 on days 1 & 2 and rituximab 375 mg/m2 day 1 of cycle 1 and 500 mg/m2 D1 cycles 2 to 6. A cycle of the regimen was Q28 days. Study treatment continued until disease progression, death, intolerable toxicity, or withdrawn consent. Stratification performed by an independent laboratory was by del17p, TP53 mutations, or mutated IGHV, and clinically by refractory (progression <6 months from completion of prior therapy) or relapsed (progression at least 6 months from completion of prior therapy) disease. Median follow-up at the time of this report was 12 months. The primary endpoint of PFS defined by standard criteria was adjudicated by an IRC. Overall survival and ORR were secondary endpoints. Crossover was not permitted at the time of CLL disease progression.
Results
Median overall survival was not reached in either arm; there was a statistically significant improvement in overall survival, after the pre-specified multiplicity adjustment, in the idelalisib arm (HR = 0.55; 95% CI: 0.36, 0.86; P = 0.008 [stratified]). As of 15 June 2015, there were 34 deaths in the idelalisib arm and 51 deaths in the placebo arm. An improvement in ORR was observed on the idelalisib vs placebo arm in all adverse-risk categories evaluated (Table).
Conclusion
Idelalisib in combination with bendamustine and rituximab is superior to bendamustine and rituximab alone, reducing the risk of death and disease progression, and increasing progression-free and overall survival. Overall response rate was also increased on the idelalisib arm. These results were consistent across pts with adverse-risk features. The safety profile was consistent with prior reported studies. This trial provides further evidence for the improved outcomes for idelalisib-based therapy in pts with relapsed/refractory CLL. This regimen represents an important new treatment option for the management of relapsed/refractory CLL, further establishing the role of idelalisib in this setting.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Phase III, PI3K
Type: Eposter Presentation
Background
Patients (pts) with relapsed/refractory CLL and adverse prognostic features (e.g., del17p/TP53mut) respond poorly to standard treatment. Here we present results of a pre-specified subgroup analysis of OS and ORR from an ongoing randomized double-blind placebo-controlled study (ClinicalTrials.gov ID: NCT01569295).
Aims
To demonstrate an improvement in OS and ORR in pts on idelalisib (IDELA) in combination with bendamustine/rituximab vs bendamustine/rituximab alone in pts with relapsed/refractory CLL and in a pre-specified pt population with adverse risk features.
Methods
Between June 2012 and August 2014, 416 pts with relapsed/refractory CLL from 180 centers in North America, Australia, New Zealand, and Europe were enrolled in this ongoing study. After informed consent, eligible pts were randomized 1:1 to idelalisib 150 mg BID (207 pts) or matching placebo (209 pts). All pts received bendamustine 70 mg/m2 on days 1 & 2 and rituximab 375 mg/m2 day 1 of cycle 1 and 500 mg/m2 D1 cycles 2 to 6. A cycle of the regimen was Q28 days. Study treatment continued until disease progression, death, intolerable toxicity, or withdrawn consent. Stratification performed by an independent laboratory was by del17p, TP53 mutations, or mutated IGHV, and clinically by refractory (progression <6 months from completion of prior therapy) or relapsed (progression at least 6 months from completion of prior therapy) disease. Median follow-up at the time of this report was 12 months. The primary endpoint of PFS defined by standard criteria was adjudicated by an IRC. Overall survival and ORR were secondary endpoints. Crossover was not permitted at the time of CLL disease progression.
Results
Median overall survival was not reached in either arm; there was a statistically significant improvement in overall survival, after the pre-specified multiplicity adjustment, in the idelalisib arm (HR = 0.55; 95% CI: 0.36, 0.86; P = 0.008 [stratified]). As of 15 June 2015, there were 34 deaths in the idelalisib arm and 51 deaths in the placebo arm. An improvement in ORR was observed on the idelalisib vs placebo arm in all adverse-risk categories evaluated (Table).
Conclusion
Idelalisib in combination with bendamustine and rituximab is superior to bendamustine and rituximab alone, reducing the risk of death and disease progression, and increasing progression-free and overall survival. Overall response rate was also increased on the idelalisib arm. These results were consistent across pts with adverse-risk features. The safety profile was consistent with prior reported studies. This trial provides further evidence for the improved outcomes for idelalisib-based therapy in pts with relapsed/refractory CLL. This regimen represents an important new treatment option for the management of relapsed/refractory CLL, further establishing the role of idelalisib in this setting.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Phase III, PI3K
{{ help_message }}
{{filter}}


