DECREASED EXPRESSION OF WNT3, A CANONICAL WNT PATHWAY LIGAND, IS FREQUENT IN CHRONIC LYMPHOCYTIC LEUKEMIA PROGRESSION AND IDENTIFIES PATIENTS WITH SHORT TREATMENT-FREE SURVIVAL IN MUTATED IGHV SUBSET
(Abstract release date: 05/19/16)
EHA Library. Poppova L. 06/09/16; 132579; E1030

Mrs. Lucie Poppova
Contributions
Contributions
Abstract
Abstract: E1030
Type: Eposter Presentation
Background
Wnt ligands drive several distinct pathways – the best explored are Wnt/β-catenin (canonical) and Wnt/Planar Cell Polarity (PCP) pathways. We have shown recently that ROR-1 driven Wnt-5a-activated WNT/PCP pathway controls migratory properties of chronic lymphocytic leukemia (CLL) cells and WNT5A positivity correlates with aggressive CLL (Janovska et al., Clin Cancer Res 2016). Several reports suggested that the canonical Wnt/β-catenin pathway, known to drive malignant transformation of multiple cell types, also participates on CLL pathogenesis. This was based on the high expression of canonical Wnt ligands and LEF1, and mutations in Wnt pathway genes. There is, however, a lack of direct evidence showing that CLL cells can respond to canonical Wnt ligands by activating β-catenin-dependent transcription. On the other hand, the observation that WNT3, encoding for the canonical Wnt ligand, is among the most up-regulated genes, supports the role of Wnt/β-catenin pathway in CLL.
Aims
To clarify the role of Wnt/β-catenin pathway in CLL by (1) detailed analysis of WNT3 expression and (2) cell-line based study of the ligand-induced pathway activation.
Methods
B-cells from CLL patients and non-malignant controls were negatively separated with RossetteSep (StemCell) or MACS kits (Miltenyi Biotec). WNT3 mRNA expression was analyzed with quantitative RT-PCR using TaqMan Gene Expression Assays (Applied Biosystems). The WNT3 expression was correlated with clinical and biological parameters. Survival analyses were performed using Kaplan-Meier curves, Cox regression model and log-rank test; cut-off was assessed in CutOff Finder web application. Wnt/β-catenin pathway activity was analyzed by the SuperTopFlash reporter system (Promega).
Results
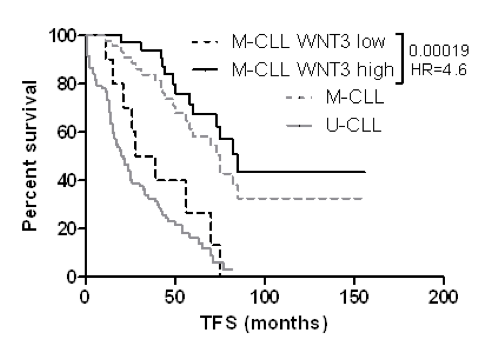
By analyzing 137 previously untreated patients we confirmed that WNT3 gene is strongly overexpressed in CLL compared to normal B-cells (P=0.0001) and that its level is higher in patients with mutated IGHV (M-CLL; P=0.047). Further, we identified low WNT3 level as a strong independent marker indicating shorter treatment-free survival (TFS) in M-CLL (P<0.0002). Time course analysis in 86 patients revealed that WNT3 declines with disease progression in a significant proportion of patients (decrease in 37%, increase in 4% of patients; P<0.0001), namely in a disease activity onset in untreated M-CLL and in a disease recurrence after therapy in patients with unmutated IGHV (U-CLL). Functional tests showed that selected lymphoid cell lines (CLL-derived MEC1 and ROR1-positive REC1) failed to respond to the ligand-induced activation of the Wnt/β-catenin pathway while bone marrow stromal cells M210B4 were able to transduce a signal to TCF/LEF-dependent transcription.
Conclusion
Our results show that despite WNT3 overexpression in CLL cells, its high levels are not associated to aggressive CLL. On the contrary, low WNT3 independently identifies patients with short TFS in M-CLL and WNT3 level decrease accompanies disease evolution in a significant proportion of patients. The data indicate that the Wnt/β-catenin pathway plays a more complex role in CLL pathogenesis than previously anticipated, opening a hypothesis that Wnt-3 ligand might be associated to early steps in CLL pathogenesis possibly via mediating interaction with the cells in the microenvironment.Supported by grants Ministry of Health of Czech Republic AZV 15-29793A, 15-30015A, 15-31834A, Ministry of Education CEITEC2020 (LQ1601), Masaryk University MUNI/A/1028/2015, EU Horizon 2020 No 692298.
Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Prognostic factor, Survival prediction, Wnt
Type: Eposter Presentation
Background
Wnt ligands drive several distinct pathways – the best explored are Wnt/β-catenin (canonical) and Wnt/Planar Cell Polarity (PCP) pathways. We have shown recently that ROR-1 driven Wnt-5a-activated WNT/PCP pathway controls migratory properties of chronic lymphocytic leukemia (CLL) cells and WNT5A positivity correlates with aggressive CLL (Janovska et al., Clin Cancer Res 2016). Several reports suggested that the canonical Wnt/β-catenin pathway, known to drive malignant transformation of multiple cell types, also participates on CLL pathogenesis. This was based on the high expression of canonical Wnt ligands and LEF1, and mutations in Wnt pathway genes. There is, however, a lack of direct evidence showing that CLL cells can respond to canonical Wnt ligands by activating β-catenin-dependent transcription. On the other hand, the observation that WNT3, encoding for the canonical Wnt ligand, is among the most up-regulated genes, supports the role of Wnt/β-catenin pathway in CLL.
Aims
To clarify the role of Wnt/β-catenin pathway in CLL by (1) detailed analysis of WNT3 expression and (2) cell-line based study of the ligand-induced pathway activation.
Methods
B-cells from CLL patients and non-malignant controls were negatively separated with RossetteSep (StemCell) or MACS kits (Miltenyi Biotec). WNT3 mRNA expression was analyzed with quantitative RT-PCR using TaqMan Gene Expression Assays (Applied Biosystems). The WNT3 expression was correlated with clinical and biological parameters. Survival analyses were performed using Kaplan-Meier curves, Cox regression model and log-rank test; cut-off was assessed in CutOff Finder web application. Wnt/β-catenin pathway activity was analyzed by the SuperTopFlash reporter system (Promega).
Results
By analyzing 137 previously untreated patients we confirmed that WNT3 gene is strongly overexpressed in CLL compared to normal B-cells (P=0.0001) and that its level is higher in patients with mutated IGHV (M-CLL; P=0.047). Further, we identified low WNT3 level as a strong independent marker indicating shorter treatment-free survival (TFS) in M-CLL (P<0.0002). Time course analysis in 86 patients revealed that WNT3 declines with disease progression in a significant proportion of patients (decrease in 37%, increase in 4% of patients; P<0.0001), namely in a disease activity onset in untreated M-CLL and in a disease recurrence after therapy in patients with unmutated IGHV (U-CLL). Functional tests showed that selected lymphoid cell lines (CLL-derived MEC1 and ROR1-positive REC1) failed to respond to the ligand-induced activation of the Wnt/β-catenin pathway while bone marrow stromal cells M210B4 were able to transduce a signal to TCF/LEF-dependent transcription.
Conclusion
Our results show that despite WNT3 overexpression in CLL cells, its high levels are not associated to aggressive CLL. On the contrary, low WNT3 independently identifies patients with short TFS in M-CLL and WNT3 level decrease accompanies disease evolution in a significant proportion of patients. The data indicate that the Wnt/β-catenin pathway plays a more complex role in CLL pathogenesis than previously anticipated, opening a hypothesis that Wnt-3 ligand might be associated to early steps in CLL pathogenesis possibly via mediating interaction with the cells in the microenvironment.Supported by grants Ministry of Health of Czech Republic AZV 15-29793A, 15-30015A, 15-31834A, Ministry of Education CEITEC2020 (LQ1601), Masaryk University MUNI/A/1028/2015, EU Horizon 2020 No 692298.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Prognostic factor, Survival prediction, Wnt
Abstract: E1030
Type: Eposter Presentation
Background
Wnt ligands drive several distinct pathways – the best explored are Wnt/β-catenin (canonical) and Wnt/Planar Cell Polarity (PCP) pathways. We have shown recently that ROR-1 driven Wnt-5a-activated WNT/PCP pathway controls migratory properties of chronic lymphocytic leukemia (CLL) cells and WNT5A positivity correlates with aggressive CLL (Janovska et al., Clin Cancer Res 2016). Several reports suggested that the canonical Wnt/β-catenin pathway, known to drive malignant transformation of multiple cell types, also participates on CLL pathogenesis. This was based on the high expression of canonical Wnt ligands and LEF1, and mutations in Wnt pathway genes. There is, however, a lack of direct evidence showing that CLL cells can respond to canonical Wnt ligands by activating β-catenin-dependent transcription. On the other hand, the observation that WNT3, encoding for the canonical Wnt ligand, is among the most up-regulated genes, supports the role of Wnt/β-catenin pathway in CLL.
Aims
To clarify the role of Wnt/β-catenin pathway in CLL by (1) detailed analysis of WNT3 expression and (2) cell-line based study of the ligand-induced pathway activation.
Methods
B-cells from CLL patients and non-malignant controls were negatively separated with RossetteSep (StemCell) or MACS kits (Miltenyi Biotec). WNT3 mRNA expression was analyzed with quantitative RT-PCR using TaqMan Gene Expression Assays (Applied Biosystems). The WNT3 expression was correlated with clinical and biological parameters. Survival analyses were performed using Kaplan-Meier curves, Cox regression model and log-rank test; cut-off was assessed in CutOff Finder web application. Wnt/β-catenin pathway activity was analyzed by the SuperTopFlash reporter system (Promega).
Results
By analyzing 137 previously untreated patients we confirmed that WNT3 gene is strongly overexpressed in CLL compared to normal B-cells (P=0.0001) and that its level is higher in patients with mutated IGHV (M-CLL; P=0.047). Further, we identified low WNT3 level as a strong independent marker indicating shorter treatment-free survival (TFS) in M-CLL (P<0.0002). Time course analysis in 86 patients revealed that WNT3 declines with disease progression in a significant proportion of patients (decrease in 37%, increase in 4% of patients; P<0.0001), namely in a disease activity onset in untreated M-CLL and in a disease recurrence after therapy in patients with unmutated IGHV (U-CLL). Functional tests showed that selected lymphoid cell lines (CLL-derived MEC1 and ROR1-positive REC1) failed to respond to the ligand-induced activation of the Wnt/β-catenin pathway while bone marrow stromal cells M210B4 were able to transduce a signal to TCF/LEF-dependent transcription.
Conclusion
Our results show that despite WNT3 overexpression in CLL cells, its high levels are not associated to aggressive CLL. On the contrary, low WNT3 independently identifies patients with short TFS in M-CLL and WNT3 level decrease accompanies disease evolution in a significant proportion of patients. The data indicate that the Wnt/β-catenin pathway plays a more complex role in CLL pathogenesis than previously anticipated, opening a hypothesis that Wnt-3 ligand might be associated to early steps in CLL pathogenesis possibly via mediating interaction with the cells in the microenvironment.Supported by grants Ministry of Health of Czech Republic AZV 15-29793A, 15-30015A, 15-31834A, Ministry of Education CEITEC2020 (LQ1601), Masaryk University MUNI/A/1028/2015, EU Horizon 2020 No 692298.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Prognostic factor, Survival prediction, Wnt
Type: Eposter Presentation
Background
Wnt ligands drive several distinct pathways – the best explored are Wnt/β-catenin (canonical) and Wnt/Planar Cell Polarity (PCP) pathways. We have shown recently that ROR-1 driven Wnt-5a-activated WNT/PCP pathway controls migratory properties of chronic lymphocytic leukemia (CLL) cells and WNT5A positivity correlates with aggressive CLL (Janovska et al., Clin Cancer Res 2016). Several reports suggested that the canonical Wnt/β-catenin pathway, known to drive malignant transformation of multiple cell types, also participates on CLL pathogenesis. This was based on the high expression of canonical Wnt ligands and LEF1, and mutations in Wnt pathway genes. There is, however, a lack of direct evidence showing that CLL cells can respond to canonical Wnt ligands by activating β-catenin-dependent transcription. On the other hand, the observation that WNT3, encoding for the canonical Wnt ligand, is among the most up-regulated genes, supports the role of Wnt/β-catenin pathway in CLL.
Aims
To clarify the role of Wnt/β-catenin pathway in CLL by (1) detailed analysis of WNT3 expression and (2) cell-line based study of the ligand-induced pathway activation.
Methods
B-cells from CLL patients and non-malignant controls were negatively separated with RossetteSep (StemCell) or MACS kits (Miltenyi Biotec). WNT3 mRNA expression was analyzed with quantitative RT-PCR using TaqMan Gene Expression Assays (Applied Biosystems). The WNT3 expression was correlated with clinical and biological parameters. Survival analyses were performed using Kaplan-Meier curves, Cox regression model and log-rank test; cut-off was assessed in CutOff Finder web application. Wnt/β-catenin pathway activity was analyzed by the SuperTopFlash reporter system (Promega).
Results
By analyzing 137 previously untreated patients we confirmed that WNT3 gene is strongly overexpressed in CLL compared to normal B-cells (P=0.0001) and that its level is higher in patients with mutated IGHV (M-CLL; P=0.047). Further, we identified low WNT3 level as a strong independent marker indicating shorter treatment-free survival (TFS) in M-CLL (P<0.0002). Time course analysis in 86 patients revealed that WNT3 declines with disease progression in a significant proportion of patients (decrease in 37%, increase in 4% of patients; P<0.0001), namely in a disease activity onset in untreated M-CLL and in a disease recurrence after therapy in patients with unmutated IGHV (U-CLL). Functional tests showed that selected lymphoid cell lines (CLL-derived MEC1 and ROR1-positive REC1) failed to respond to the ligand-induced activation of the Wnt/β-catenin pathway while bone marrow stromal cells M210B4 were able to transduce a signal to TCF/LEF-dependent transcription.
Conclusion
Our results show that despite WNT3 overexpression in CLL cells, its high levels are not associated to aggressive CLL. On the contrary, low WNT3 independently identifies patients with short TFS in M-CLL and WNT3 level decrease accompanies disease evolution in a significant proportion of patients. The data indicate that the Wnt/β-catenin pathway plays a more complex role in CLL pathogenesis than previously anticipated, opening a hypothesis that Wnt-3 ligand might be associated to early steps in CLL pathogenesis possibly via mediating interaction with the cells in the microenvironment.Supported by grants Ministry of Health of Czech Republic AZV 15-29793A, 15-30015A, 15-31834A, Ministry of Education CEITEC2020 (LQ1601), Masaryk University MUNI/A/1028/2015, EU Horizon 2020 No 692298.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Prognostic factor, Survival prediction, Wnt
{{ help_message }}
{{filter}}


