CD49D IS A BETTER PREDICTOR OF OVERALL SURVIVAL THAN THE NOVEL RECURRENT MUTATIONS IN CHRONIC LYMPHOCYTIC LEUKEMIA: RESULTS FROM AN ITALIAN MULTI-CENTER COHORT
(Abstract release date: 05/19/16)
EHA Library. Gattei V. 06/09/16; 132565; E1016

Dr. Valter Gattei
Contributions
Contributions
Abstract
Abstract: E1016
Type: Eposter Presentation
Background
CD49d, the alpha-chain of VLA-4 integrin, was identified among the strongest predictors of overall survival (OS) in chronic lymphocytic leukemia (CLL), along with IGHV status and the 17p deletion (Bulian et al, JCO, 2014). In addition to TP53, the clinical relevance of mutations of NOTCH1, SF3B1 and BIRC3 recently emerged.
Aims
To test the clinical relevance of CD49d in subgroups defined by NOTCH1, SF3B1, BIRC3 alterations.
Methods
The cohort was of 778 unselected CLL (treated cases, n=356, median follow-up 80 months with 173 deaths), all characterized for CD49d expression (CD49dhigh, ≥30% positive cells by flow cytometry, n=229), IGHV status (unmutated, UM, n=262), karyotype abnormalities (5% cut-off, 13q-, n=308; +12, n=103; 11q-, n=64), at diagnosis, along with TP53 mutations/deletions (hereinafter, disruption, n=84, including 58 17p-), NOTCH1 mutations (n=81), SF3B1 mutations (n=54), BIRC3 disruption (n=59). Recurrent mutations were investigated by Sanger sequencing before therapy (at diagnosis in 65% of cases).
Results
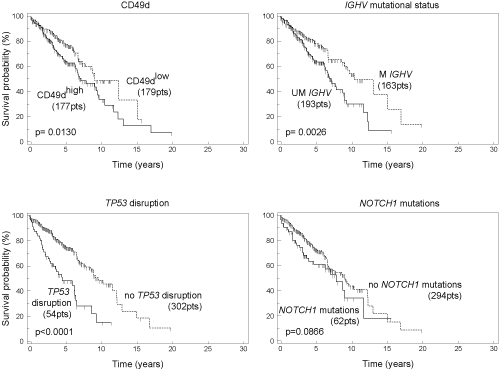
i) CD49dhigh associated with age ≥65 years (p=0.0001), Rai stage ≥I (p<0.0001), UM IGHV status (p>0.0001), absence of 13q- (p=0.0001), presence of +12 (p<0.0001), NOTCH1 mutations (p<0.0001). ii) By univariate analysis, CD49dhigh had impact as OS predictor (hazard ratio/confidence interval, HR/CI=2.62/1.94-3.54; p<0.0001). In multivariate analysis, CD49dhigh was confirmed as independent prognosticator (HR/CI=1.88/1.36-2.60, p<0.0001), along with age≥65 years (HR/CI=3.90/2.68-5.67), Rai stage ≥I (HR/CI=1.80/1.30-2.51, p=0.0005), UM IGHV (HR/CI=1.84/1.31-2.60, p=0.0005), 11q- (HR/CI=2.21/1.39-3.51, p=0.0008), TP53 disruption (HR/CI=3.65/2.15-5.30, p<0.0001), NOTCH1 mutations (HR/CI=1.79/1.17-2.73, p=0.0068). Conversely, +12, SF3B1 mutations, BIRC3 disruption were all excluded from the final model. iii) The variable importance (VIMP) of biological markers as OS predictors was evaluated by a random forests approach. CD49d (VIMP=0.0410) was ranked among the most important OS predictors, followed by IGHV status (VIMP=0.0388), TP53 disruption (VIMP=0.0352) and NOTCH1 mutations (VIMP=0.0168); conversely, SF3B1 mutations (VIMP=0.0002) and BIRC3 disruption (VIMP=-0.0024) had no importance as OS predictors. iv) Post-treatment survival was evaluated to explore the impact of prognosticators as therapy response predictors. CD49dhigh, UM IGHV, TP53 disruption but not NOTCH1 mutations were able to split cases into groups with significant different treatment responses (see Figure). This observation was confirmed by multivariate analysis where CD49dhigh (HR/CI=1.55/1.08-2.23, p=0.0192), UM IGHV (HR/CI=1.70/1.17-2.49, p=0.0060), TP53 disruption (HR/CI=2.67/1.79-4.00, p<0.0001) were maintained and NOTCH1 mutations were excluded. iv) In Rai stage 0 cases (n=389), CD49dhigh had impact as Time-to-First-Treatment predictor (HR/CI, 2.68/1.78-4.05, p<0.0001) by univariate analysis. In multivariate analysis, CD49dhigh again emerged as independent prognosticator (HR/CI=1.61/1.01-2.56, p=0.0442), along with UM IGHV (HR=2.66/1.72-4.12, p<0.0001), +12 (HR/CI=3.36/2.00-5.62, p<0.0001), TP53 disruption (HR/CI=1.97/1.03-3.78, p=0.0419), BIRC3 disruption (HR/CI=2.70/1.24-5.89, p=0.0127), whereas age, NOTCH1 mutations, 13q-, 11q-, were excluded.
Conclusion
CD49d, along with IGHV status, is the most powerful independent negative OS prognosticator in CLL also when NOTCH1, SF3B1, BIRC3 alterations were evaluated. CD49d, IGHV status and TP53 disruption may also have a role as therapy response predictors.
Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin, Prognostic factor
Type: Eposter Presentation
Background
CD49d, the alpha-chain of VLA-4 integrin, was identified among the strongest predictors of overall survival (OS) in chronic lymphocytic leukemia (CLL), along with IGHV status and the 17p deletion (Bulian et al, JCO, 2014). In addition to TP53, the clinical relevance of mutations of NOTCH1, SF3B1 and BIRC3 recently emerged.
Aims
To test the clinical relevance of CD49d in subgroups defined by NOTCH1, SF3B1, BIRC3 alterations.
Methods
The cohort was of 778 unselected CLL (treated cases, n=356, median follow-up 80 months with 173 deaths), all characterized for CD49d expression (CD49dhigh, ≥30% positive cells by flow cytometry, n=229), IGHV status (unmutated, UM, n=262), karyotype abnormalities (5% cut-off, 13q-, n=308; +12, n=103; 11q-, n=64), at diagnosis, along with TP53 mutations/deletions (hereinafter, disruption, n=84, including 58 17p-), NOTCH1 mutations (n=81), SF3B1 mutations (n=54), BIRC3 disruption (n=59). Recurrent mutations were investigated by Sanger sequencing before therapy (at diagnosis in 65% of cases).
Results
i) CD49dhigh associated with age ≥65 years (p=0.0001), Rai stage ≥I (p<0.0001), UM IGHV status (p>0.0001), absence of 13q- (p=0.0001), presence of +12 (p<0.0001), NOTCH1 mutations (p<0.0001). ii) By univariate analysis, CD49dhigh had impact as OS predictor (hazard ratio/confidence interval, HR/CI=2.62/1.94-3.54; p<0.0001). In multivariate analysis, CD49dhigh was confirmed as independent prognosticator (HR/CI=1.88/1.36-2.60, p<0.0001), along with age≥65 years (HR/CI=3.90/2.68-5.67), Rai stage ≥I (HR/CI=1.80/1.30-2.51, p=0.0005), UM IGHV (HR/CI=1.84/1.31-2.60, p=0.0005), 11q- (HR/CI=2.21/1.39-3.51, p=0.0008), TP53 disruption (HR/CI=3.65/2.15-5.30, p<0.0001), NOTCH1 mutations (HR/CI=1.79/1.17-2.73, p=0.0068). Conversely, +12, SF3B1 mutations, BIRC3 disruption were all excluded from the final model. iii) The variable importance (VIMP) of biological markers as OS predictors was evaluated by a random forests approach. CD49d (VIMP=0.0410) was ranked among the most important OS predictors, followed by IGHV status (VIMP=0.0388), TP53 disruption (VIMP=0.0352) and NOTCH1 mutations (VIMP=0.0168); conversely, SF3B1 mutations (VIMP=0.0002) and BIRC3 disruption (VIMP=-0.0024) had no importance as OS predictors. iv) Post-treatment survival was evaluated to explore the impact of prognosticators as therapy response predictors. CD49dhigh, UM IGHV, TP53 disruption but not NOTCH1 mutations were able to split cases into groups with significant different treatment responses (see Figure). This observation was confirmed by multivariate analysis where CD49dhigh (HR/CI=1.55/1.08-2.23, p=0.0192), UM IGHV (HR/CI=1.70/1.17-2.49, p=0.0060), TP53 disruption (HR/CI=2.67/1.79-4.00, p<0.0001) were maintained and NOTCH1 mutations were excluded. iv) In Rai stage 0 cases (n=389), CD49dhigh had impact as Time-to-First-Treatment predictor (HR/CI, 2.68/1.78-4.05, p<0.0001) by univariate analysis. In multivariate analysis, CD49dhigh again emerged as independent prognosticator (HR/CI=1.61/1.01-2.56, p=0.0442), along with UM IGHV (HR=2.66/1.72-4.12, p<0.0001), +12 (HR/CI=3.36/2.00-5.62, p<0.0001), TP53 disruption (HR/CI=1.97/1.03-3.78, p=0.0419), BIRC3 disruption (HR/CI=2.70/1.24-5.89, p=0.0127), whereas age, NOTCH1 mutations, 13q-, 11q-, were excluded.
Conclusion
CD49d, along with IGHV status, is the most powerful independent negative OS prognosticator in CLL also when NOTCH1, SF3B1, BIRC3 alterations were evaluated. CD49d, IGHV status and TP53 disruption may also have a role as therapy response predictors.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin, Prognostic factor
Abstract: E1016
Type: Eposter Presentation
Background
CD49d, the alpha-chain of VLA-4 integrin, was identified among the strongest predictors of overall survival (OS) in chronic lymphocytic leukemia (CLL), along with IGHV status and the 17p deletion (Bulian et al, JCO, 2014). In addition to TP53, the clinical relevance of mutations of NOTCH1, SF3B1 and BIRC3 recently emerged.
Aims
To test the clinical relevance of CD49d in subgroups defined by NOTCH1, SF3B1, BIRC3 alterations.
Methods
The cohort was of 778 unselected CLL (treated cases, n=356, median follow-up 80 months with 173 deaths), all characterized for CD49d expression (CD49dhigh, ≥30% positive cells by flow cytometry, n=229), IGHV status (unmutated, UM, n=262), karyotype abnormalities (5% cut-off, 13q-, n=308; +12, n=103; 11q-, n=64), at diagnosis, along with TP53 mutations/deletions (hereinafter, disruption, n=84, including 58 17p-), NOTCH1 mutations (n=81), SF3B1 mutations (n=54), BIRC3 disruption (n=59). Recurrent mutations were investigated by Sanger sequencing before therapy (at diagnosis in 65% of cases).
Results
i) CD49dhigh associated with age ≥65 years (p=0.0001), Rai stage ≥I (p<0.0001), UM IGHV status (p>0.0001), absence of 13q- (p=0.0001), presence of +12 (p<0.0001), NOTCH1 mutations (p<0.0001). ii) By univariate analysis, CD49dhigh had impact as OS predictor (hazard ratio/confidence interval, HR/CI=2.62/1.94-3.54; p<0.0001). In multivariate analysis, CD49dhigh was confirmed as independent prognosticator (HR/CI=1.88/1.36-2.60, p<0.0001), along with age≥65 years (HR/CI=3.90/2.68-5.67), Rai stage ≥I (HR/CI=1.80/1.30-2.51, p=0.0005), UM IGHV (HR/CI=1.84/1.31-2.60, p=0.0005), 11q- (HR/CI=2.21/1.39-3.51, p=0.0008), TP53 disruption (HR/CI=3.65/2.15-5.30, p<0.0001), NOTCH1 mutations (HR/CI=1.79/1.17-2.73, p=0.0068). Conversely, +12, SF3B1 mutations, BIRC3 disruption were all excluded from the final model. iii) The variable importance (VIMP) of biological markers as OS predictors was evaluated by a random forests approach. CD49d (VIMP=0.0410) was ranked among the most important OS predictors, followed by IGHV status (VIMP=0.0388), TP53 disruption (VIMP=0.0352) and NOTCH1 mutations (VIMP=0.0168); conversely, SF3B1 mutations (VIMP=0.0002) and BIRC3 disruption (VIMP=-0.0024) had no importance as OS predictors. iv) Post-treatment survival was evaluated to explore the impact of prognosticators as therapy response predictors. CD49dhigh, UM IGHV, TP53 disruption but not NOTCH1 mutations were able to split cases into groups with significant different treatment responses (see Figure). This observation was confirmed by multivariate analysis where CD49dhigh (HR/CI=1.55/1.08-2.23, p=0.0192), UM IGHV (HR/CI=1.70/1.17-2.49, p=0.0060), TP53 disruption (HR/CI=2.67/1.79-4.00, p<0.0001) were maintained and NOTCH1 mutations were excluded. iv) In Rai stage 0 cases (n=389), CD49dhigh had impact as Time-to-First-Treatment predictor (HR/CI, 2.68/1.78-4.05, p<0.0001) by univariate analysis. In multivariate analysis, CD49dhigh again emerged as independent prognosticator (HR/CI=1.61/1.01-2.56, p=0.0442), along with UM IGHV (HR=2.66/1.72-4.12, p<0.0001), +12 (HR/CI=3.36/2.00-5.62, p<0.0001), TP53 disruption (HR/CI=1.97/1.03-3.78, p=0.0419), BIRC3 disruption (HR/CI=2.70/1.24-5.89, p=0.0127), whereas age, NOTCH1 mutations, 13q-, 11q-, were excluded.
Conclusion
CD49d, along with IGHV status, is the most powerful independent negative OS prognosticator in CLL also when NOTCH1, SF3B1, BIRC3 alterations were evaluated. CD49d, IGHV status and TP53 disruption may also have a role as therapy response predictors.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin, Prognostic factor
Type: Eposter Presentation
Background
CD49d, the alpha-chain of VLA-4 integrin, was identified among the strongest predictors of overall survival (OS) in chronic lymphocytic leukemia (CLL), along with IGHV status and the 17p deletion (Bulian et al, JCO, 2014). In addition to TP53, the clinical relevance of mutations of NOTCH1, SF3B1 and BIRC3 recently emerged.
Aims
To test the clinical relevance of CD49d in subgroups defined by NOTCH1, SF3B1, BIRC3 alterations.
Methods
The cohort was of 778 unselected CLL (treated cases, n=356, median follow-up 80 months with 173 deaths), all characterized for CD49d expression (CD49dhigh, ≥30% positive cells by flow cytometry, n=229), IGHV status (unmutated, UM, n=262), karyotype abnormalities (5% cut-off, 13q-, n=308; +12, n=103; 11q-, n=64), at diagnosis, along with TP53 mutations/deletions (hereinafter, disruption, n=84, including 58 17p-), NOTCH1 mutations (n=81), SF3B1 mutations (n=54), BIRC3 disruption (n=59). Recurrent mutations were investigated by Sanger sequencing before therapy (at diagnosis in 65% of cases).
Results
i) CD49dhigh associated with age ≥65 years (p=0.0001), Rai stage ≥I (p<0.0001), UM IGHV status (p>0.0001), absence of 13q- (p=0.0001), presence of +12 (p<0.0001), NOTCH1 mutations (p<0.0001). ii) By univariate analysis, CD49dhigh had impact as OS predictor (hazard ratio/confidence interval, HR/CI=2.62/1.94-3.54; p<0.0001). In multivariate analysis, CD49dhigh was confirmed as independent prognosticator (HR/CI=1.88/1.36-2.60, p<0.0001), along with age≥65 years (HR/CI=3.90/2.68-5.67), Rai stage ≥I (HR/CI=1.80/1.30-2.51, p=0.0005), UM IGHV (HR/CI=1.84/1.31-2.60, p=0.0005), 11q- (HR/CI=2.21/1.39-3.51, p=0.0008), TP53 disruption (HR/CI=3.65/2.15-5.30, p<0.0001), NOTCH1 mutations (HR/CI=1.79/1.17-2.73, p=0.0068). Conversely, +12, SF3B1 mutations, BIRC3 disruption were all excluded from the final model. iii) The variable importance (VIMP) of biological markers as OS predictors was evaluated by a random forests approach. CD49d (VIMP=0.0410) was ranked among the most important OS predictors, followed by IGHV status (VIMP=0.0388), TP53 disruption (VIMP=0.0352) and NOTCH1 mutations (VIMP=0.0168); conversely, SF3B1 mutations (VIMP=0.0002) and BIRC3 disruption (VIMP=-0.0024) had no importance as OS predictors. iv) Post-treatment survival was evaluated to explore the impact of prognosticators as therapy response predictors. CD49dhigh, UM IGHV, TP53 disruption but not NOTCH1 mutations were able to split cases into groups with significant different treatment responses (see Figure). This observation was confirmed by multivariate analysis where CD49dhigh (HR/CI=1.55/1.08-2.23, p=0.0192), UM IGHV (HR/CI=1.70/1.17-2.49, p=0.0060), TP53 disruption (HR/CI=2.67/1.79-4.00, p<0.0001) were maintained and NOTCH1 mutations were excluded. iv) In Rai stage 0 cases (n=389), CD49dhigh had impact as Time-to-First-Treatment predictor (HR/CI, 2.68/1.78-4.05, p<0.0001) by univariate analysis. In multivariate analysis, CD49dhigh again emerged as independent prognosticator (HR/CI=1.61/1.01-2.56, p=0.0442), along with UM IGHV (HR=2.66/1.72-4.12, p<0.0001), +12 (HR/CI=3.36/2.00-5.62, p<0.0001), TP53 disruption (HR/CI=1.97/1.03-3.78, p=0.0419), BIRC3 disruption (HR/CI=2.70/1.24-5.89, p=0.0127), whereas age, NOTCH1 mutations, 13q-, 11q-, were excluded.
Conclusion
CD49d, along with IGHV status, is the most powerful independent negative OS prognosticator in CLL also when NOTCH1, SF3B1, BIRC3 alterations were evaluated. CD49d, IGHV status and TP53 disruption may also have a role as therapy response predictors.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Integrin, Prognostic factor
{{ help_message }}
{{filter}}


