THE PI3K DELTA SELECTIVE INHIBITOR, IDELALISIB, MODULATES CYTOKINE PRODUCTION IN INNATE IMMUNE CELLS STIMULATED THROUGH TOLL-LIKE RECEPTORS
(Abstract release date: 05/19/16)
EHA Library. Tannheimer S. 06/09/16; 132562; E1013
Disclosure(s): Gilead Sciences employees and stock shareholders

Dr. Stacey Tannheimer
Contributions
Contributions
Abstract
Abstract: E1013
Type: Eposter Presentation
Background
Idelalisib (IDELA) is an oral, selective PI3Kd inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia (CLL). A subset of patients develop severe diarrhea/colitis which requires interruption of IDELA therapy and in some instances, corticosteroid treatment (Coutre et al., Leuk Lymphoma, 2015). Toll-like receptors (TLR) play a critical role in innate immunity by detecting pathogen associated molecular patterns (PAMPs). Responses to signaling through TLR drive not only an immediate innate cell response to pathogens, but also enable cells of the innate immune system to shape an adaptive immune response. PI3Kd has been shown to modulate signal transduction through TLR4 and TLR1/2 in murine bone marrow derived dendritic cells (Aksoy et al., Nat Imm, 2012). Additionally, PI3Kd has been shown to modulate TLR2, TLR4, TLR5, and TLR9 signaling in murine bone marrow derived macrophages (Plevy et al., Gastroenterology, 2010). Mice with a targeted inactivating mutation in the p110d subunit of PI3Kd spontaneously develop colitis at 8 weeks of age, and bone marrow macrophages from these mice secreted significantly more inflammatory cytokines (Plevy et al., Gastroenterology, 2010).
Aims
Evaluate the effects of clinically relevant concentrations of IDELA on TLR signaling in human monocyte derived macrophages (MDMs) as a potential mechanism contributing to clinically observed adverse events.
Methods
We utilized MDMs skewed to either a M1 or M2 phenotype by culture for six days in GM-CSF or M-CSF, respectively. MDMs stimulated with a TLR1/2 (PAM3CSK4), TLR4 (LPS), or TLR5 (flagellin) agonist in the presence of clinically relevant concentrations of IDELA were examined for cytokine production at early and late time points (MesoScale Discovery). Additionally, nCounter Human Inflammation Kit V2 (NanoString) was used to assess the effects on gene expression and Peggy Sue (Protein Simple) was used to analyze changes in protein expression on MDMs.
Results
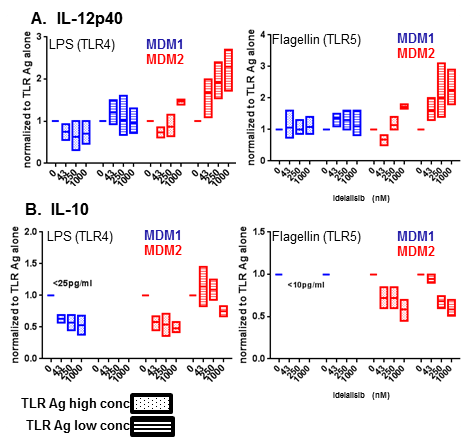
Cytokine analysis revealed that both MDM1 and MDM2 cells produced inflammatory IL-12p40 when stimulated with TLR agonists. However, IL-12p40 production by MDM2 cells when stimulated with TLR1/2, TLR4 or TLR5 agonists was significantly increased in the presence of IDELA (Fig. 1A). Inflammatory cytokine upregulation was not seen in MDM1 cells. MDM2 cells produced immunosuppressive IL-10 in response to TLR1/2, TLR4 or TLR5 agonist stimulation. The IL-10 production was inhibited in the presence of IDELA (Fig. 1B). MDM1 cells produced little to no IL-10 in this context. IDELA exerted a differential effect on gene expression in MDM2 versus MDM1 cells. CXCR2 ligands were upregulated in MDM2 cells in the presence of IDELA plus TLR agonists to a much greater extent than MDM1 cells, while the anti-inflammatory IL-1RA was downregulated.
Conclusion
The immunological challenge of the mucosal environment of the intestine is maintaining a balance between a tolerogenic state with the commensal microbiota and an ability to aggressively respond to potential pathogens. Under normal conditions, TLR signaling by enteric bacteria is protective (Rakoff-Nahoum et al., Cell, 2004). Here we show that IDELA modulates TLR signaling in MDMs, resulting in MDM2 cells producing higher amounts of inflammatory cytokines, and less immunosuppressive IL-10. This dysregulation of the tolerogenic cytokine balance and subsequent perturbations of the innate/adaptive immune system is consistent with proinflammatory cytokine imbalance which may result in colitis in PI3Kd deficient animal models.
Session topic: E-poster
Keyword(s): Cytokine, LPS, Macrophage, PI3K
Type: Eposter Presentation
Background
Idelalisib (IDELA) is an oral, selective PI3Kd inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia (CLL). A subset of patients develop severe diarrhea/colitis which requires interruption of IDELA therapy and in some instances, corticosteroid treatment (Coutre et al., Leuk Lymphoma, 2015). Toll-like receptors (TLR) play a critical role in innate immunity by detecting pathogen associated molecular patterns (PAMPs). Responses to signaling through TLR drive not only an immediate innate cell response to pathogens, but also enable cells of the innate immune system to shape an adaptive immune response. PI3Kd has been shown to modulate signal transduction through TLR4 and TLR1/2 in murine bone marrow derived dendritic cells (Aksoy et al., Nat Imm, 2012). Additionally, PI3Kd has been shown to modulate TLR2, TLR4, TLR5, and TLR9 signaling in murine bone marrow derived macrophages (Plevy et al., Gastroenterology, 2010). Mice with a targeted inactivating mutation in the p110d subunit of PI3Kd spontaneously develop colitis at 8 weeks of age, and bone marrow macrophages from these mice secreted significantly more inflammatory cytokines (Plevy et al., Gastroenterology, 2010).
Aims
Evaluate the effects of clinically relevant concentrations of IDELA on TLR signaling in human monocyte derived macrophages (MDMs) as a potential mechanism contributing to clinically observed adverse events.
Methods
We utilized MDMs skewed to either a M1 or M2 phenotype by culture for six days in GM-CSF or M-CSF, respectively. MDMs stimulated with a TLR1/2 (PAM3CSK4), TLR4 (LPS), or TLR5 (flagellin) agonist in the presence of clinically relevant concentrations of IDELA were examined for cytokine production at early and late time points (MesoScale Discovery). Additionally, nCounter Human Inflammation Kit V2 (NanoString) was used to assess the effects on gene expression and Peggy Sue (Protein Simple) was used to analyze changes in protein expression on MDMs.
Results
Cytokine analysis revealed that both MDM1 and MDM2 cells produced inflammatory IL-12p40 when stimulated with TLR agonists. However, IL-12p40 production by MDM2 cells when stimulated with TLR1/2, TLR4 or TLR5 agonists was significantly increased in the presence of IDELA (Fig. 1A). Inflammatory cytokine upregulation was not seen in MDM1 cells. MDM2 cells produced immunosuppressive IL-10 in response to TLR1/2, TLR4 or TLR5 agonist stimulation. The IL-10 production was inhibited in the presence of IDELA (Fig. 1B). MDM1 cells produced little to no IL-10 in this context. IDELA exerted a differential effect on gene expression in MDM2 versus MDM1 cells. CXCR2 ligands were upregulated in MDM2 cells in the presence of IDELA plus TLR agonists to a much greater extent than MDM1 cells, while the anti-inflammatory IL-1RA was downregulated.
Conclusion
The immunological challenge of the mucosal environment of the intestine is maintaining a balance between a tolerogenic state with the commensal microbiota and an ability to aggressively respond to potential pathogens. Under normal conditions, TLR signaling by enteric bacteria is protective (Rakoff-Nahoum et al., Cell, 2004). Here we show that IDELA modulates TLR signaling in MDMs, resulting in MDM2 cells producing higher amounts of inflammatory cytokines, and less immunosuppressive IL-10. This dysregulation of the tolerogenic cytokine balance and subsequent perturbations of the innate/adaptive immune system is consistent with proinflammatory cytokine imbalance which may result in colitis in PI3Kd deficient animal models.

Session topic: E-poster
Keyword(s): Cytokine, LPS, Macrophage, PI3K
Abstract: E1013
Type: Eposter Presentation
Background
Idelalisib (IDELA) is an oral, selective PI3Kd inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia (CLL). A subset of patients develop severe diarrhea/colitis which requires interruption of IDELA therapy and in some instances, corticosteroid treatment (Coutre et al., Leuk Lymphoma, 2015). Toll-like receptors (TLR) play a critical role in innate immunity by detecting pathogen associated molecular patterns (PAMPs). Responses to signaling through TLR drive not only an immediate innate cell response to pathogens, but also enable cells of the innate immune system to shape an adaptive immune response. PI3Kd has been shown to modulate signal transduction through TLR4 and TLR1/2 in murine bone marrow derived dendritic cells (Aksoy et al., Nat Imm, 2012). Additionally, PI3Kd has been shown to modulate TLR2, TLR4, TLR5, and TLR9 signaling in murine bone marrow derived macrophages (Plevy et al., Gastroenterology, 2010). Mice with a targeted inactivating mutation in the p110d subunit of PI3Kd spontaneously develop colitis at 8 weeks of age, and bone marrow macrophages from these mice secreted significantly more inflammatory cytokines (Plevy et al., Gastroenterology, 2010).
Aims
Evaluate the effects of clinically relevant concentrations of IDELA on TLR signaling in human monocyte derived macrophages (MDMs) as a potential mechanism contributing to clinically observed adverse events.
Methods
We utilized MDMs skewed to either a M1 or M2 phenotype by culture for six days in GM-CSF or M-CSF, respectively. MDMs stimulated with a TLR1/2 (PAM3CSK4), TLR4 (LPS), or TLR5 (flagellin) agonist in the presence of clinically relevant concentrations of IDELA were examined for cytokine production at early and late time points (MesoScale Discovery). Additionally, nCounter Human Inflammation Kit V2 (NanoString) was used to assess the effects on gene expression and Peggy Sue (Protein Simple) was used to analyze changes in protein expression on MDMs.
Results
Cytokine analysis revealed that both MDM1 and MDM2 cells produced inflammatory IL-12p40 when stimulated with TLR agonists. However, IL-12p40 production by MDM2 cells when stimulated with TLR1/2, TLR4 or TLR5 agonists was significantly increased in the presence of IDELA (Fig. 1A). Inflammatory cytokine upregulation was not seen in MDM1 cells. MDM2 cells produced immunosuppressive IL-10 in response to TLR1/2, TLR4 or TLR5 agonist stimulation. The IL-10 production was inhibited in the presence of IDELA (Fig. 1B). MDM1 cells produced little to no IL-10 in this context. IDELA exerted a differential effect on gene expression in MDM2 versus MDM1 cells. CXCR2 ligands were upregulated in MDM2 cells in the presence of IDELA plus TLR agonists to a much greater extent than MDM1 cells, while the anti-inflammatory IL-1RA was downregulated.
Conclusion
The immunological challenge of the mucosal environment of the intestine is maintaining a balance between a tolerogenic state with the commensal microbiota and an ability to aggressively respond to potential pathogens. Under normal conditions, TLR signaling by enteric bacteria is protective (Rakoff-Nahoum et al., Cell, 2004). Here we show that IDELA modulates TLR signaling in MDMs, resulting in MDM2 cells producing higher amounts of inflammatory cytokines, and less immunosuppressive IL-10. This dysregulation of the tolerogenic cytokine balance and subsequent perturbations of the innate/adaptive immune system is consistent with proinflammatory cytokine imbalance which may result in colitis in PI3Kd deficient animal models.

Session topic: E-poster
Keyword(s): Cytokine, LPS, Macrophage, PI3K
Type: Eposter Presentation
Background
Idelalisib (IDELA) is an oral, selective PI3Kd inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia (CLL). A subset of patients develop severe diarrhea/colitis which requires interruption of IDELA therapy and in some instances, corticosteroid treatment (Coutre et al., Leuk Lymphoma, 2015). Toll-like receptors (TLR) play a critical role in innate immunity by detecting pathogen associated molecular patterns (PAMPs). Responses to signaling through TLR drive not only an immediate innate cell response to pathogens, but also enable cells of the innate immune system to shape an adaptive immune response. PI3Kd has been shown to modulate signal transduction through TLR4 and TLR1/2 in murine bone marrow derived dendritic cells (Aksoy et al., Nat Imm, 2012). Additionally, PI3Kd has been shown to modulate TLR2, TLR4, TLR5, and TLR9 signaling in murine bone marrow derived macrophages (Plevy et al., Gastroenterology, 2010). Mice with a targeted inactivating mutation in the p110d subunit of PI3Kd spontaneously develop colitis at 8 weeks of age, and bone marrow macrophages from these mice secreted significantly more inflammatory cytokines (Plevy et al., Gastroenterology, 2010).
Aims
Evaluate the effects of clinically relevant concentrations of IDELA on TLR signaling in human monocyte derived macrophages (MDMs) as a potential mechanism contributing to clinically observed adverse events.
Methods
We utilized MDMs skewed to either a M1 or M2 phenotype by culture for six days in GM-CSF or M-CSF, respectively. MDMs stimulated with a TLR1/2 (PAM3CSK4), TLR4 (LPS), or TLR5 (flagellin) agonist in the presence of clinically relevant concentrations of IDELA were examined for cytokine production at early and late time points (MesoScale Discovery). Additionally, nCounter Human Inflammation Kit V2 (NanoString) was used to assess the effects on gene expression and Peggy Sue (Protein Simple) was used to analyze changes in protein expression on MDMs.
Results
Cytokine analysis revealed that both MDM1 and MDM2 cells produced inflammatory IL-12p40 when stimulated with TLR agonists. However, IL-12p40 production by MDM2 cells when stimulated with TLR1/2, TLR4 or TLR5 agonists was significantly increased in the presence of IDELA (Fig. 1A). Inflammatory cytokine upregulation was not seen in MDM1 cells. MDM2 cells produced immunosuppressive IL-10 in response to TLR1/2, TLR4 or TLR5 agonist stimulation. The IL-10 production was inhibited in the presence of IDELA (Fig. 1B). MDM1 cells produced little to no IL-10 in this context. IDELA exerted a differential effect on gene expression in MDM2 versus MDM1 cells. CXCR2 ligands were upregulated in MDM2 cells in the presence of IDELA plus TLR agonists to a much greater extent than MDM1 cells, while the anti-inflammatory IL-1RA was downregulated.
Conclusion
The immunological challenge of the mucosal environment of the intestine is maintaining a balance between a tolerogenic state with the commensal microbiota and an ability to aggressively respond to potential pathogens. Under normal conditions, TLR signaling by enteric bacteria is protective (Rakoff-Nahoum et al., Cell, 2004). Here we show that IDELA modulates TLR signaling in MDMs, resulting in MDM2 cells producing higher amounts of inflammatory cytokines, and less immunosuppressive IL-10. This dysregulation of the tolerogenic cytokine balance and subsequent perturbations of the innate/adaptive immune system is consistent with proinflammatory cytokine imbalance which may result in colitis in PI3Kd deficient animal models.

Session topic: E-poster
Keyword(s): Cytokine, LPS, Macrophage, PI3K
{{ help_message }}
{{filter}}


