EFFICACY AND SAFETY OF DEFIBROTIDE FOR TREATMENT OF HEPATIC VENO-OCCLUSIVE DISEASE/SINUSOIDAL OBSTRUCTION SYNDROME (VOD/SOS) IN ADULT AND PEDIATRIC PATIENTS FOLLOWING CHEMOTHERAPY
(Abstract release date: 05/19/16)
EHA Library. RICHARDSON P. 06/09/16; 132561; E1012

Dr. PAUL RICHARDSON
Contributions
Contributions
Abstract
Abstract: E1012
Type: Eposter Presentation
Background
Veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is typically thought of as an unpredictable and potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT) conditioning, but also may occur following primary chemotherapy (CT) without HSCT. Severe hepatic VOD/SOS (associated with multi-organ dysfunction [MOD]) may be associated with >80% mortality. Endothelial cell (EC) damage is a critical pathophysiologic factor; preclinical data suggest that defibrotide (DF) stabilizes ECs with direct, as well as EC-mediated, restoration of the thrombo-fibrinolytic balance. DF is approved in the European Union for treatment of severe hepatic VOD/SOS in adult and pediatric patients post-HSCT. In the United States (US), a new drug application was accepted for priority review in 2015. DF has been available in the US on an investigational basis via a treatment protocol (T-IND) study.
Aims
To assess the efficacy of DF for treatment of VOD/SOS in pediatric and adult subgroups following primary CT with MOD or without MOD (off-label), from the T-IND program.
Methods
Eligibility for the T-IND program initially required VOD/SOS diagnosed by Baltimore criteria, with MOD, following HSCT. The protocol was amended to include patients with VOD/SOS without MOD, following CT, or as diagnosed by modified Seattle criteria (using a 5% weight gain threshold). All patients provided informed consent and received open-label defibrotide as a 2-hour intravenous infusion at 6.25 mg/kg every 6 hours (25 mg/kg/day) for a recommended ≥21 days. Survival at Day+100 following CT was measured using Kaplan-Meier (KM) estimates.
Results
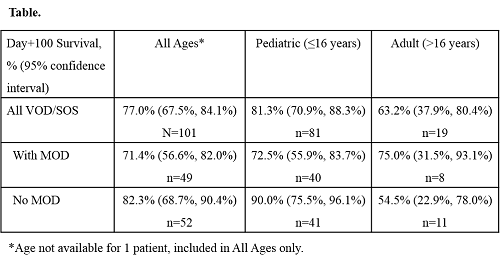
Among 857 T-IND patients with VOD/SOS who received ≥1 dose of DF as of April 18, 2015, 101 (11.8%) had undergone primary CT (without HSCT), of whom 54.5% were male, 66.3% were white, and median (range) age was 7.0 (0, 69.0) years. This group included 81 (80.2%) pediatric patients (aged ≤16 years; 59 [72.8%] aged 2–11 years) and 19 (18.8%) adults (aged >16 years) (age not available for 1 patient); 48 (47.5%) patients had MOD (40 [49.4%] pediatric patients and 8 [42.1%] adults), and 52 (51.5%) did not have MOD (41 [50.6%] pediatric patients and 11 [57.9%] adults). For all patients and for the subset without MOD, the most commonly used chemotherapy agents included vincristine, cytarabine, and cyclophosphamide. KM-estimated Day+100 survival is reported in the Table.Overall, 62 (61.4%) patients reported ≥1 adverse event (AE), including 22 (21.8%) with AEs judged at least possibly related to DF, most commonly (≥3.0%) hypotension (4.0%) and nausea (3.0%). Two deaths (pleural hemorrhage and pulmonary hemorrhage plus hypotension) were possibly related to DF.
Conclusion
KM-estimated survival rates at Day+100 post-CT for patients treated with DF were 81% in pediatric patients and 63% in adults, which were clinically encouraging findings. As has been observed in post-HSCT patients, pediatric patients may have higher survival rates than adults post-CT. DF was generally well tolerated in this study and had a safety profile consistent with previous studies. Support: Jazz Pharmaceuticals
Session topic: E-poster
Keyword(s): Chemotherapy, Defibrotide, Veno-occlusive disease
Type: Eposter Presentation
Background
Veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is typically thought of as an unpredictable and potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT) conditioning, but also may occur following primary chemotherapy (CT) without HSCT. Severe hepatic VOD/SOS (associated with multi-organ dysfunction [MOD]) may be associated with >80% mortality. Endothelial cell (EC) damage is a critical pathophysiologic factor; preclinical data suggest that defibrotide (DF) stabilizes ECs with direct, as well as EC-mediated, restoration of the thrombo-fibrinolytic balance. DF is approved in the European Union for treatment of severe hepatic VOD/SOS in adult and pediatric patients post-HSCT. In the United States (US), a new drug application was accepted for priority review in 2015. DF has been available in the US on an investigational basis via a treatment protocol (T-IND) study.
Aims
To assess the efficacy of DF for treatment of VOD/SOS in pediatric and adult subgroups following primary CT with MOD or without MOD (off-label), from the T-IND program.
Methods
Eligibility for the T-IND program initially required VOD/SOS diagnosed by Baltimore criteria, with MOD, following HSCT. The protocol was amended to include patients with VOD/SOS without MOD, following CT, or as diagnosed by modified Seattle criteria (using a 5% weight gain threshold). All patients provided informed consent and received open-label defibrotide as a 2-hour intravenous infusion at 6.25 mg/kg every 6 hours (25 mg/kg/day) for a recommended ≥21 days. Survival at Day+100 following CT was measured using Kaplan-Meier (KM) estimates.
Results
Among 857 T-IND patients with VOD/SOS who received ≥1 dose of DF as of April 18, 2015, 101 (11.8%) had undergone primary CT (without HSCT), of whom 54.5% were male, 66.3% were white, and median (range) age was 7.0 (0, 69.0) years. This group included 81 (80.2%) pediatric patients (aged ≤16 years; 59 [72.8%] aged 2–11 years) and 19 (18.8%) adults (aged >16 years) (age not available for 1 patient); 48 (47.5%) patients had MOD (40 [49.4%] pediatric patients and 8 [42.1%] adults), and 52 (51.5%) did not have MOD (41 [50.6%] pediatric patients and 11 [57.9%] adults). For all patients and for the subset without MOD, the most commonly used chemotherapy agents included vincristine, cytarabine, and cyclophosphamide. KM-estimated Day+100 survival is reported in the Table.Overall, 62 (61.4%) patients reported ≥1 adverse event (AE), including 22 (21.8%) with AEs judged at least possibly related to DF, most commonly (≥3.0%) hypotension (4.0%) and nausea (3.0%). Two deaths (pleural hemorrhage and pulmonary hemorrhage plus hypotension) were possibly related to DF.
Conclusion
KM-estimated survival rates at Day+100 post-CT for patients treated with DF were 81% in pediatric patients and 63% in adults, which were clinically encouraging findings. As has been observed in post-HSCT patients, pediatric patients may have higher survival rates than adults post-CT. DF was generally well tolerated in this study and had a safety profile consistent with previous studies. Support: Jazz Pharmaceuticals

Session topic: E-poster
Keyword(s): Chemotherapy, Defibrotide, Veno-occlusive disease
Abstract: E1012
Type: Eposter Presentation
Background
Veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is typically thought of as an unpredictable and potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT) conditioning, but also may occur following primary chemotherapy (CT) without HSCT. Severe hepatic VOD/SOS (associated with multi-organ dysfunction [MOD]) may be associated with >80% mortality. Endothelial cell (EC) damage is a critical pathophysiologic factor; preclinical data suggest that defibrotide (DF) stabilizes ECs with direct, as well as EC-mediated, restoration of the thrombo-fibrinolytic balance. DF is approved in the European Union for treatment of severe hepatic VOD/SOS in adult and pediatric patients post-HSCT. In the United States (US), a new drug application was accepted for priority review in 2015. DF has been available in the US on an investigational basis via a treatment protocol (T-IND) study.
Aims
To assess the efficacy of DF for treatment of VOD/SOS in pediatric and adult subgroups following primary CT with MOD or without MOD (off-label), from the T-IND program.
Methods
Eligibility for the T-IND program initially required VOD/SOS diagnosed by Baltimore criteria, with MOD, following HSCT. The protocol was amended to include patients with VOD/SOS without MOD, following CT, or as diagnosed by modified Seattle criteria (using a 5% weight gain threshold). All patients provided informed consent and received open-label defibrotide as a 2-hour intravenous infusion at 6.25 mg/kg every 6 hours (25 mg/kg/day) for a recommended ≥21 days. Survival at Day+100 following CT was measured using Kaplan-Meier (KM) estimates.
Results
Among 857 T-IND patients with VOD/SOS who received ≥1 dose of DF as of April 18, 2015, 101 (11.8%) had undergone primary CT (without HSCT), of whom 54.5% were male, 66.3% were white, and median (range) age was 7.0 (0, 69.0) years. This group included 81 (80.2%) pediatric patients (aged ≤16 years; 59 [72.8%] aged 2–11 years) and 19 (18.8%) adults (aged >16 years) (age not available for 1 patient); 48 (47.5%) patients had MOD (40 [49.4%] pediatric patients and 8 [42.1%] adults), and 52 (51.5%) did not have MOD (41 [50.6%] pediatric patients and 11 [57.9%] adults). For all patients and for the subset without MOD, the most commonly used chemotherapy agents included vincristine, cytarabine, and cyclophosphamide. KM-estimated Day+100 survival is reported in the Table.Overall, 62 (61.4%) patients reported ≥1 adverse event (AE), including 22 (21.8%) with AEs judged at least possibly related to DF, most commonly (≥3.0%) hypotension (4.0%) and nausea (3.0%). Two deaths (pleural hemorrhage and pulmonary hemorrhage plus hypotension) were possibly related to DF.
Conclusion
KM-estimated survival rates at Day+100 post-CT for patients treated with DF were 81% in pediatric patients and 63% in adults, which were clinically encouraging findings. As has been observed in post-HSCT patients, pediatric patients may have higher survival rates than adults post-CT. DF was generally well tolerated in this study and had a safety profile consistent with previous studies. Support: Jazz Pharmaceuticals

Session topic: E-poster
Keyword(s): Chemotherapy, Defibrotide, Veno-occlusive disease
Type: Eposter Presentation
Background
Veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is typically thought of as an unpredictable and potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT) conditioning, but also may occur following primary chemotherapy (CT) without HSCT. Severe hepatic VOD/SOS (associated with multi-organ dysfunction [MOD]) may be associated with >80% mortality. Endothelial cell (EC) damage is a critical pathophysiologic factor; preclinical data suggest that defibrotide (DF) stabilizes ECs with direct, as well as EC-mediated, restoration of the thrombo-fibrinolytic balance. DF is approved in the European Union for treatment of severe hepatic VOD/SOS in adult and pediatric patients post-HSCT. In the United States (US), a new drug application was accepted for priority review in 2015. DF has been available in the US on an investigational basis via a treatment protocol (T-IND) study.
Aims
To assess the efficacy of DF for treatment of VOD/SOS in pediatric and adult subgroups following primary CT with MOD or without MOD (off-label), from the T-IND program.
Methods
Eligibility for the T-IND program initially required VOD/SOS diagnosed by Baltimore criteria, with MOD, following HSCT. The protocol was amended to include patients with VOD/SOS without MOD, following CT, or as diagnosed by modified Seattle criteria (using a 5% weight gain threshold). All patients provided informed consent and received open-label defibrotide as a 2-hour intravenous infusion at 6.25 mg/kg every 6 hours (25 mg/kg/day) for a recommended ≥21 days. Survival at Day+100 following CT was measured using Kaplan-Meier (KM) estimates.
Results
Among 857 T-IND patients with VOD/SOS who received ≥1 dose of DF as of April 18, 2015, 101 (11.8%) had undergone primary CT (without HSCT), of whom 54.5% were male, 66.3% were white, and median (range) age was 7.0 (0, 69.0) years. This group included 81 (80.2%) pediatric patients (aged ≤16 years; 59 [72.8%] aged 2–11 years) and 19 (18.8%) adults (aged >16 years) (age not available for 1 patient); 48 (47.5%) patients had MOD (40 [49.4%] pediatric patients and 8 [42.1%] adults), and 52 (51.5%) did not have MOD (41 [50.6%] pediatric patients and 11 [57.9%] adults). For all patients and for the subset without MOD, the most commonly used chemotherapy agents included vincristine, cytarabine, and cyclophosphamide. KM-estimated Day+100 survival is reported in the Table.Overall, 62 (61.4%) patients reported ≥1 adverse event (AE), including 22 (21.8%) with AEs judged at least possibly related to DF, most commonly (≥3.0%) hypotension (4.0%) and nausea (3.0%). Two deaths (pleural hemorrhage and pulmonary hemorrhage plus hypotension) were possibly related to DF.
Conclusion
KM-estimated survival rates at Day+100 post-CT for patients treated with DF were 81% in pediatric patients and 63% in adults, which were clinically encouraging findings. As has been observed in post-HSCT patients, pediatric patients may have higher survival rates than adults post-CT. DF was generally well tolerated in this study and had a safety profile consistent with previous studies. Support: Jazz Pharmaceuticals

Session topic: E-poster
Keyword(s): Chemotherapy, Defibrotide, Veno-occlusive disease
{{ help_message }}
{{filter}}


