DOSE-ADJUSTED ETOPOSIDE-PREDNISONE-VINCRISTINE-CYCLOPHOSPHAMIDE-DOXORUBICIN-RITUXIMAB (DA-EPOCH-R) FOR PRIMARY MEDIASTINAL B-CELL LYMPHOMA (PMBCL) AND OTHER VERY HIGH-RISK OR ADVANCED LYMPHOMAS
(Abstract release date: 05/19/16)
EHA Library. Carobolante F. 06/09/16; 132528; E979

Dr. Francesca Carobolante
Contributions
Contributions
Abstract
Abstract: E979
Type: Eposter Presentation
Background
Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab (DA-EPOCH-R) is highly effective in PMBCL (Wilson W et al. NEJM 2013;368:1408) and is adopted in very aggressive lymphomas with encouraging results. While it is commonly used in US, experience in European countries is limited.
Aims
DA-EPOCH-R was used at Venice hospitals to treat adult patients with PMBCL and aggressive lymphoma variants associated with a poor response to R-CHOP.
Methods
DA–EPOCH-R consists of 6-8 cycles q21d with escalating doses of etoposide, cyclophosphamide and doxorubicin. Etoposide, vincristine (fixed dose 0.4 mg/m2/d) and doxorubicin are given by continuous IV infusion over 96 hours (dd 1-4). Drug level 1 is with etoposide 50, doxorubicin 10 and cyclophosphamide (d5) 750 mg/m2/d. A 20% dose escalation after each cycle and up to level 6 is prescribed to patients who do not develop a neutropenia <500/µl. The dose is reduced by 20% if neutropenia lasts >1 week or platelets fall <25.000/µl. Concomitant drugs are prednisone 60 mg/m2/bd (dd 1-5), rituximab 375 mg/m2 d1, and G-CSF from d6. Complete remission (CR, CRu [unconfirmed]) is defined according to standard criteria and PET/CT assessment after a minimum of 4 cycles.
Results
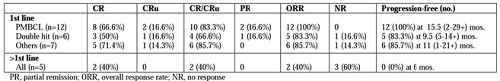
Thirty-three patients were treated between august 2013 and january 2016. Twenty-eight patients were treated frontline for PMBCL (n=14), non-GCB/highly proliferative (Ki67 ≥80%) diffuse large B-cell lymphoma (DLBCL) (n=5), double-hit DLBCL (n=6), plasmablastic lymphoma (n=2, one without R), and EBV+ lymphomatoid granulomatosis (n=1). Five patients were treated at salvage after 1-2 prior lines for DLBCL (n=3), Burkitt lymphoma (n=1) and Hodgkin disease (n=1, without R). Median patient age was 57.5 years (range 17-74 years), 61% were male, 73% had ECOG PS >1, 64% had stage III-IV disease and 58% had intermediate-high/high IPI. Altogether, the 33 patients received 158 courses, without change in dose level in 5 salvage patients. Twenty-six of 28 untreated patients had 2 or more cycles and are evaluable for dose level increments. Dose level could not be increased in 8 patients (31%), reached level 2 in 5 (19%), level 3 in 7 (27%), level 4 in 5 (19%), level 5 in 1 (4%) and level 6 in none. Toxicity consisted of severe neutropenia and thrombocytopenia in 67% and 10% of the cycles, respectively, neutropenic fever with admission to hospital (n=5, 15%), vincristine-related neuropathy (n=3, 9%), and thromboembolism (n=3, 9%). Due to poor clinical conditions, treatment was stopped after cycle 1 in a DLBCL patient (aged 65) with concurrent lung cancer, and switched to R-CHOP after 2 and 4 cycles in 2 patients (aged 74 and 61, respectively) with double-hit DLBCL, both remaining well and alive. Twenty-five patients completing 4 courses with PET/CT-based restaging are evaluable for response (Table). CR and ORR rate was high in patients treated frontline, particularly in PMBCL (ORR and PFS 100%). Response rate was appreciable in double-hit and very aggressive lymphomas as well, but was instead poor in patients treated at salvage.
Conclusion
DA-EPOCH-R proved globally feasible and safe in this aged patient population, and highly active in PMBCL as observed in the original study. The small patient number and the limited follow-up period do not allow definite conclusions, although the regimen would be useful in PMBCL (avoiding mediastinal irradiation) and potentially useful in very aggressive B-cell lymphomas at risk of failing standard R-CHOP therapy.
Session topic: E-poster
Keyword(s): Dose escalation, Lymphoma therapy
Type: Eposter Presentation
Background
Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab (DA-EPOCH-R) is highly effective in PMBCL (Wilson W et al. NEJM 2013;368:1408) and is adopted in very aggressive lymphomas with encouraging results. While it is commonly used in US, experience in European countries is limited.
Aims
DA-EPOCH-R was used at Venice hospitals to treat adult patients with PMBCL and aggressive lymphoma variants associated with a poor response to R-CHOP.
Methods
DA–EPOCH-R consists of 6-8 cycles q21d with escalating doses of etoposide, cyclophosphamide and doxorubicin. Etoposide, vincristine (fixed dose 0.4 mg/m2/d) and doxorubicin are given by continuous IV infusion over 96 hours (dd 1-4). Drug level 1 is with etoposide 50, doxorubicin 10 and cyclophosphamide (d5) 750 mg/m2/d. A 20% dose escalation after each cycle and up to level 6 is prescribed to patients who do not develop a neutropenia <500/µl. The dose is reduced by 20% if neutropenia lasts >1 week or platelets fall <25.000/µl. Concomitant drugs are prednisone 60 mg/m2/bd (dd 1-5), rituximab 375 mg/m2 d1, and G-CSF from d6. Complete remission (CR, CRu [unconfirmed]) is defined according to standard criteria and PET/CT assessment after a minimum of 4 cycles.
Results
Thirty-three patients were treated between august 2013 and january 2016. Twenty-eight patients were treated frontline for PMBCL (n=14), non-GCB/highly proliferative (Ki67 ≥80%) diffuse large B-cell lymphoma (DLBCL) (n=5), double-hit DLBCL (n=6), plasmablastic lymphoma (n=2, one without R), and EBV+ lymphomatoid granulomatosis (n=1). Five patients were treated at salvage after 1-2 prior lines for DLBCL (n=3), Burkitt lymphoma (n=1) and Hodgkin disease (n=1, without R). Median patient age was 57.5 years (range 17-74 years), 61% were male, 73% had ECOG PS >1, 64% had stage III-IV disease and 58% had intermediate-high/high IPI. Altogether, the 33 patients received 158 courses, without change in dose level in 5 salvage patients. Twenty-six of 28 untreated patients had 2 or more cycles and are evaluable for dose level increments. Dose level could not be increased in 8 patients (31%), reached level 2 in 5 (19%), level 3 in 7 (27%), level 4 in 5 (19%), level 5 in 1 (4%) and level 6 in none. Toxicity consisted of severe neutropenia and thrombocytopenia in 67% and 10% of the cycles, respectively, neutropenic fever with admission to hospital (n=5, 15%), vincristine-related neuropathy (n=3, 9%), and thromboembolism (n=3, 9%). Due to poor clinical conditions, treatment was stopped after cycle 1 in a DLBCL patient (aged 65) with concurrent lung cancer, and switched to R-CHOP after 2 and 4 cycles in 2 patients (aged 74 and 61, respectively) with double-hit DLBCL, both remaining well and alive. Twenty-five patients completing 4 courses with PET/CT-based restaging are evaluable for response (Table). CR and ORR rate was high in patients treated frontline, particularly in PMBCL (ORR and PFS 100%). Response rate was appreciable in double-hit and very aggressive lymphomas as well, but was instead poor in patients treated at salvage.
Conclusion
DA-EPOCH-R proved globally feasible and safe in this aged patient population, and highly active in PMBCL as observed in the original study. The small patient number and the limited follow-up period do not allow definite conclusions, although the regimen would be useful in PMBCL (avoiding mediastinal irradiation) and potentially useful in very aggressive B-cell lymphomas at risk of failing standard R-CHOP therapy.

Session topic: E-poster
Keyword(s): Dose escalation, Lymphoma therapy
Abstract: E979
Type: Eposter Presentation
Background
Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab (DA-EPOCH-R) is highly effective in PMBCL (Wilson W et al. NEJM 2013;368:1408) and is adopted in very aggressive lymphomas with encouraging results. While it is commonly used in US, experience in European countries is limited.
Aims
DA-EPOCH-R was used at Venice hospitals to treat adult patients with PMBCL and aggressive lymphoma variants associated with a poor response to R-CHOP.
Methods
DA–EPOCH-R consists of 6-8 cycles q21d with escalating doses of etoposide, cyclophosphamide and doxorubicin. Etoposide, vincristine (fixed dose 0.4 mg/m2/d) and doxorubicin are given by continuous IV infusion over 96 hours (dd 1-4). Drug level 1 is with etoposide 50, doxorubicin 10 and cyclophosphamide (d5) 750 mg/m2/d. A 20% dose escalation after each cycle and up to level 6 is prescribed to patients who do not develop a neutropenia <500/µl. The dose is reduced by 20% if neutropenia lasts >1 week or platelets fall <25.000/µl. Concomitant drugs are prednisone 60 mg/m2/bd (dd 1-5), rituximab 375 mg/m2 d1, and G-CSF from d6. Complete remission (CR, CRu [unconfirmed]) is defined according to standard criteria and PET/CT assessment after a minimum of 4 cycles.
Results
Thirty-three patients were treated between august 2013 and january 2016. Twenty-eight patients were treated frontline for PMBCL (n=14), non-GCB/highly proliferative (Ki67 ≥80%) diffuse large B-cell lymphoma (DLBCL) (n=5), double-hit DLBCL (n=6), plasmablastic lymphoma (n=2, one without R), and EBV+ lymphomatoid granulomatosis (n=1). Five patients were treated at salvage after 1-2 prior lines for DLBCL (n=3), Burkitt lymphoma (n=1) and Hodgkin disease (n=1, without R). Median patient age was 57.5 years (range 17-74 years), 61% were male, 73% had ECOG PS >1, 64% had stage III-IV disease and 58% had intermediate-high/high IPI. Altogether, the 33 patients received 158 courses, without change in dose level in 5 salvage patients. Twenty-six of 28 untreated patients had 2 or more cycles and are evaluable for dose level increments. Dose level could not be increased in 8 patients (31%), reached level 2 in 5 (19%), level 3 in 7 (27%), level 4 in 5 (19%), level 5 in 1 (4%) and level 6 in none. Toxicity consisted of severe neutropenia and thrombocytopenia in 67% and 10% of the cycles, respectively, neutropenic fever with admission to hospital (n=5, 15%), vincristine-related neuropathy (n=3, 9%), and thromboembolism (n=3, 9%). Due to poor clinical conditions, treatment was stopped after cycle 1 in a DLBCL patient (aged 65) with concurrent lung cancer, and switched to R-CHOP after 2 and 4 cycles in 2 patients (aged 74 and 61, respectively) with double-hit DLBCL, both remaining well and alive. Twenty-five patients completing 4 courses with PET/CT-based restaging are evaluable for response (Table). CR and ORR rate was high in patients treated frontline, particularly in PMBCL (ORR and PFS 100%). Response rate was appreciable in double-hit and very aggressive lymphomas as well, but was instead poor in patients treated at salvage.
Conclusion
DA-EPOCH-R proved globally feasible and safe in this aged patient population, and highly active in PMBCL as observed in the original study. The small patient number and the limited follow-up period do not allow definite conclusions, although the regimen would be useful in PMBCL (avoiding mediastinal irradiation) and potentially useful in very aggressive B-cell lymphomas at risk of failing standard R-CHOP therapy.

Session topic: E-poster
Keyword(s): Dose escalation, Lymphoma therapy
Type: Eposter Presentation
Background
Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab (DA-EPOCH-R) is highly effective in PMBCL (Wilson W et al. NEJM 2013;368:1408) and is adopted in very aggressive lymphomas with encouraging results. While it is commonly used in US, experience in European countries is limited.
Aims
DA-EPOCH-R was used at Venice hospitals to treat adult patients with PMBCL and aggressive lymphoma variants associated with a poor response to R-CHOP.
Methods
DA–EPOCH-R consists of 6-8 cycles q21d with escalating doses of etoposide, cyclophosphamide and doxorubicin. Etoposide, vincristine (fixed dose 0.4 mg/m2/d) and doxorubicin are given by continuous IV infusion over 96 hours (dd 1-4). Drug level 1 is with etoposide 50, doxorubicin 10 and cyclophosphamide (d5) 750 mg/m2/d. A 20% dose escalation after each cycle and up to level 6 is prescribed to patients who do not develop a neutropenia <500/µl. The dose is reduced by 20% if neutropenia lasts >1 week or platelets fall <25.000/µl. Concomitant drugs are prednisone 60 mg/m2/bd (dd 1-5), rituximab 375 mg/m2 d1, and G-CSF from d6. Complete remission (CR, CRu [unconfirmed]) is defined according to standard criteria and PET/CT assessment after a minimum of 4 cycles.
Results
Thirty-three patients were treated between august 2013 and january 2016. Twenty-eight patients were treated frontline for PMBCL (n=14), non-GCB/highly proliferative (Ki67 ≥80%) diffuse large B-cell lymphoma (DLBCL) (n=5), double-hit DLBCL (n=6), plasmablastic lymphoma (n=2, one without R), and EBV+ lymphomatoid granulomatosis (n=1). Five patients were treated at salvage after 1-2 prior lines for DLBCL (n=3), Burkitt lymphoma (n=1) and Hodgkin disease (n=1, without R). Median patient age was 57.5 years (range 17-74 years), 61% were male, 73% had ECOG PS >1, 64% had stage III-IV disease and 58% had intermediate-high/high IPI. Altogether, the 33 patients received 158 courses, without change in dose level in 5 salvage patients. Twenty-six of 28 untreated patients had 2 or more cycles and are evaluable for dose level increments. Dose level could not be increased in 8 patients (31%), reached level 2 in 5 (19%), level 3 in 7 (27%), level 4 in 5 (19%), level 5 in 1 (4%) and level 6 in none. Toxicity consisted of severe neutropenia and thrombocytopenia in 67% and 10% of the cycles, respectively, neutropenic fever with admission to hospital (n=5, 15%), vincristine-related neuropathy (n=3, 9%), and thromboembolism (n=3, 9%). Due to poor clinical conditions, treatment was stopped after cycle 1 in a DLBCL patient (aged 65) with concurrent lung cancer, and switched to R-CHOP after 2 and 4 cycles in 2 patients (aged 74 and 61, respectively) with double-hit DLBCL, both remaining well and alive. Twenty-five patients completing 4 courses with PET/CT-based restaging are evaluable for response (Table). CR and ORR rate was high in patients treated frontline, particularly in PMBCL (ORR and PFS 100%). Response rate was appreciable in double-hit and very aggressive lymphomas as well, but was instead poor in patients treated at salvage.
Conclusion
DA-EPOCH-R proved globally feasible and safe in this aged patient population, and highly active in PMBCL as observed in the original study. The small patient number and the limited follow-up period do not allow definite conclusions, although the regimen would be useful in PMBCL (avoiding mediastinal irradiation) and potentially useful in very aggressive B-cell lymphomas at risk of failing standard R-CHOP therapy.

Session topic: E-poster
Keyword(s): Dose escalation, Lymphoma therapy
{{ help_message }}
{{filter}}


