OUTCOME FOR PATIENTS WITH RELAPSED/REFRACTORY LYMPHOMA TREATED WITH GEMCITABINE AND OXALIPLATIN WITH OR WITHOUT RITUXIMAB
(Abstract release date: 05/19/16)
EHA Library. Dhanapal V. 06/09/16; 132527; E978

Dr. Vijayavalli Dhanapal
Contributions
Contributions
Abstract
Abstract: E978
Type: Eposter Presentation
Background
Relapsed/refractory aggressive lymphoma remains challenging to treat. There is no standard salvage chemotherapy regimen. Studies suggest that the outpatient combination of Gemcitabine, Oxaliplatin (Gem-Ox) with Rituximab (R) achieves similar response rates (RR) with less toxicity than intensive inpatient regimens1.
Aims
To review outcomes for patients with refractory/relapsed aggressive lymphoma treated with Gem/Ox+/-R.
Methods
This retrospective study analysed clinical data on patients with relapsed/refractory aggressive lymphoma treated with Gem/Ox with or without R between 2010 and 2015 at 3 teaching hospitals in London, UK. The treatment schedule was Gemcitabine 1000mg/m2 and Oxaliplatin 100mg/m2 +/- Rituximab 375 mg/m2 on day 1 and repeated every 15 days for up to 8 cycles.
Results
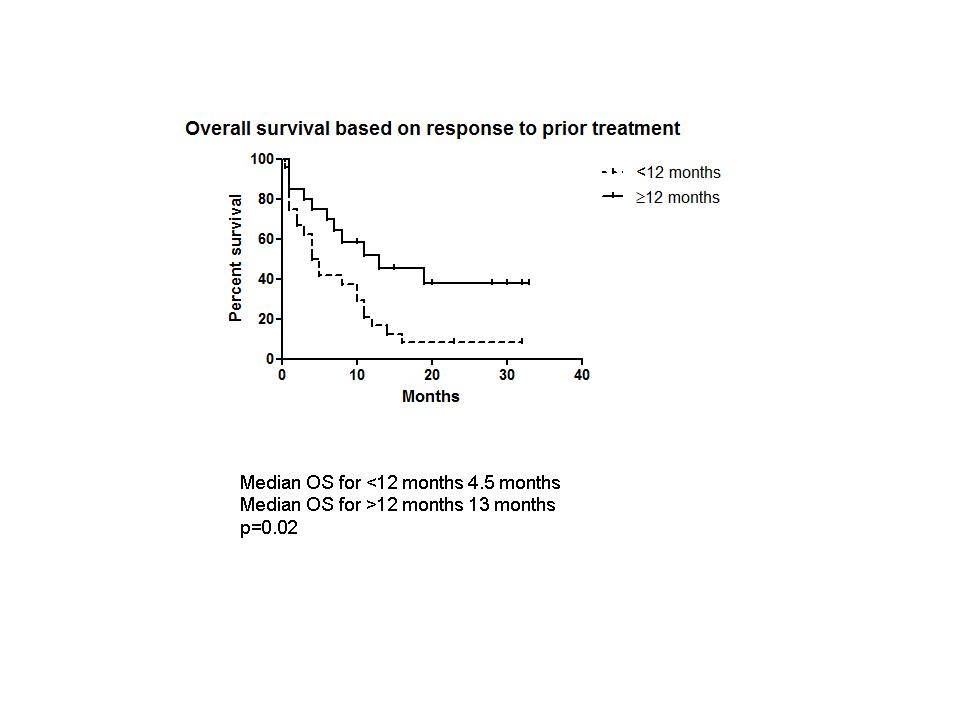
44 patients, 26 male, with a median age of 66 years (range 22-83) were included. 30 (68%) patients had DLBCL; 27 had received prior R. 6 had T-cell lymphoma, 3 Hodgkin lymphoma and 5 had high grade transformation of low grade B-cell lymphoma. The median number of prior treatments was 1 (range 1-6). 7 patients had received an autologous transplant, 1 of whom had also received an allogeneic transplant. Remission duration (RD) to the prior treatment regimen was <12 months in 26 (59%) patients. 26 (75%) patients received R with Gem-Ox. The median number of cycles received was 4 (range 1-6). Response was assessed by CT scan in 19 PET/CT in 19, MRI in 2 and by clinical evaluation in 4. The overall response was 43% (n=19) with a complete response (CR) of 29% (n=13). CR was achieved in 11/18 (61%) patients with a prior RD ≥12 months compared to 2/26 (8%) patients whose prior RD was <12 months (p=0.0002). 27/44 (61%) patients were transplant eligible, 7 of whom had received a prior autologous transplant. The overall response in transplant eligible patients was 44 % (n=12) 8/27 (30%) went on to receive a stem cell transplant; 4 autologous and 4 allogeneic transplants. At a median follow up of 6 months (range 0.5-33), the median overall survival (OS) is 8 months. For the 19 responding patients the median RD has not been reached. Median OS was significantly better in patients who had had a ≥12 month response to their prior treatment regimen; 13 vs 4.5 months, p=0.02 (Fig 1). Toxicity data were available for 36 patients. 17(47%) patients experienced grade 3/4 haematological toxicity. 8 (22%) had at least one febrile episode requiring hospitalisation. There were 4 (11%) grade 3/4 non-haematological toxicities, including diarrhoea, vomiting, peripheral neuropathy and bowel perforation. Dose reductions were required in 5 patients. 33 (75%) patients have died, 26 (60%) from lymphoma. Other causes of death include 1 old age, 2 post transplant graft vs host disease, 1 treatment-related sepsis 3 unknown.
Conclusion
In this study of ‘real world’ patients, Gem-Ox+/-R is a well-tolerated, outpatient regimen achieving good response rates in patients with chemo-responsive disease. Responses are comparable to more toxic, inpatient, platinum-based regimens. It can also successfully bridge patients to stem cell transplantation. Best responses are achieved in patients who have had a prior remission of at least 1 year.
Session topic: E-poster
Keyword(s): Gemcitabine, High-grade non-Hodgkins-lymphoma
Type: Eposter Presentation
Background
Relapsed/refractory aggressive lymphoma remains challenging to treat. There is no standard salvage chemotherapy regimen. Studies suggest that the outpatient combination of Gemcitabine, Oxaliplatin (Gem-Ox) with Rituximab (R) achieves similar response rates (RR) with less toxicity than intensive inpatient regimens1.
Aims
To review outcomes for patients with refractory/relapsed aggressive lymphoma treated with Gem/Ox+/-R.
Methods
This retrospective study analysed clinical data on patients with relapsed/refractory aggressive lymphoma treated with Gem/Ox with or without R between 2010 and 2015 at 3 teaching hospitals in London, UK. The treatment schedule was Gemcitabine 1000mg/m2 and Oxaliplatin 100mg/m2 +/- Rituximab 375 mg/m2 on day 1 and repeated every 15 days for up to 8 cycles.
Results
44 patients, 26 male, with a median age of 66 years (range 22-83) were included. 30 (68%) patients had DLBCL; 27 had received prior R. 6 had T-cell lymphoma, 3 Hodgkin lymphoma and 5 had high grade transformation of low grade B-cell lymphoma. The median number of prior treatments was 1 (range 1-6). 7 patients had received an autologous transplant, 1 of whom had also received an allogeneic transplant. Remission duration (RD) to the prior treatment regimen was <12 months in 26 (59%) patients. 26 (75%) patients received R with Gem-Ox. The median number of cycles received was 4 (range 1-6). Response was assessed by CT scan in 19 PET/CT in 19, MRI in 2 and by clinical evaluation in 4. The overall response was 43% (n=19) with a complete response (CR) of 29% (n=13). CR was achieved in 11/18 (61%) patients with a prior RD ≥12 months compared to 2/26 (8%) patients whose prior RD was <12 months (p=0.0002). 27/44 (61%) patients were transplant eligible, 7 of whom had received a prior autologous transplant. The overall response in transplant eligible patients was 44 % (n=12) 8/27 (30%) went on to receive a stem cell transplant; 4 autologous and 4 allogeneic transplants. At a median follow up of 6 months (range 0.5-33), the median overall survival (OS) is 8 months. For the 19 responding patients the median RD has not been reached. Median OS was significantly better in patients who had had a ≥12 month response to their prior treatment regimen; 13 vs 4.5 months, p=0.02 (Fig 1). Toxicity data were available for 36 patients. 17(47%) patients experienced grade 3/4 haematological toxicity. 8 (22%) had at least one febrile episode requiring hospitalisation. There were 4 (11%) grade 3/4 non-haematological toxicities, including diarrhoea, vomiting, peripheral neuropathy and bowel perforation. Dose reductions were required in 5 patients. 33 (75%) patients have died, 26 (60%) from lymphoma. Other causes of death include 1 old age, 2 post transplant graft vs host disease, 1 treatment-related sepsis 3 unknown.
Conclusion
In this study of ‘real world’ patients, Gem-Ox+/-R is a well-tolerated, outpatient regimen achieving good response rates in patients with chemo-responsive disease. Responses are comparable to more toxic, inpatient, platinum-based regimens. It can also successfully bridge patients to stem cell transplantation. Best responses are achieved in patients who have had a prior remission of at least 1 year.

Session topic: E-poster
Keyword(s): Gemcitabine, High-grade non-Hodgkins-lymphoma
Abstract: E978
Type: Eposter Presentation
Background
Relapsed/refractory aggressive lymphoma remains challenging to treat. There is no standard salvage chemotherapy regimen. Studies suggest that the outpatient combination of Gemcitabine, Oxaliplatin (Gem-Ox) with Rituximab (R) achieves similar response rates (RR) with less toxicity than intensive inpatient regimens1.
Aims
To review outcomes for patients with refractory/relapsed aggressive lymphoma treated with Gem/Ox+/-R.
Methods
This retrospective study analysed clinical data on patients with relapsed/refractory aggressive lymphoma treated with Gem/Ox with or without R between 2010 and 2015 at 3 teaching hospitals in London, UK. The treatment schedule was Gemcitabine 1000mg/m2 and Oxaliplatin 100mg/m2 +/- Rituximab 375 mg/m2 on day 1 and repeated every 15 days for up to 8 cycles.
Results
44 patients, 26 male, with a median age of 66 years (range 22-83) were included. 30 (68%) patients had DLBCL; 27 had received prior R. 6 had T-cell lymphoma, 3 Hodgkin lymphoma and 5 had high grade transformation of low grade B-cell lymphoma. The median number of prior treatments was 1 (range 1-6). 7 patients had received an autologous transplant, 1 of whom had also received an allogeneic transplant. Remission duration (RD) to the prior treatment regimen was <12 months in 26 (59%) patients. 26 (75%) patients received R with Gem-Ox. The median number of cycles received was 4 (range 1-6). Response was assessed by CT scan in 19 PET/CT in 19, MRI in 2 and by clinical evaluation in 4. The overall response was 43% (n=19) with a complete response (CR) of 29% (n=13). CR was achieved in 11/18 (61%) patients with a prior RD ≥12 months compared to 2/26 (8%) patients whose prior RD was <12 months (p=0.0002). 27/44 (61%) patients were transplant eligible, 7 of whom had received a prior autologous transplant. The overall response in transplant eligible patients was 44 % (n=12) 8/27 (30%) went on to receive a stem cell transplant; 4 autologous and 4 allogeneic transplants. At a median follow up of 6 months (range 0.5-33), the median overall survival (OS) is 8 months. For the 19 responding patients the median RD has not been reached. Median OS was significantly better in patients who had had a ≥12 month response to their prior treatment regimen; 13 vs 4.5 months, p=0.02 (Fig 1). Toxicity data were available for 36 patients. 17(47%) patients experienced grade 3/4 haematological toxicity. 8 (22%) had at least one febrile episode requiring hospitalisation. There were 4 (11%) grade 3/4 non-haematological toxicities, including diarrhoea, vomiting, peripheral neuropathy and bowel perforation. Dose reductions were required in 5 patients. 33 (75%) patients have died, 26 (60%) from lymphoma. Other causes of death include 1 old age, 2 post transplant graft vs host disease, 1 treatment-related sepsis 3 unknown.
Conclusion
In this study of ‘real world’ patients, Gem-Ox+/-R is a well-tolerated, outpatient regimen achieving good response rates in patients with chemo-responsive disease. Responses are comparable to more toxic, inpatient, platinum-based regimens. It can also successfully bridge patients to stem cell transplantation. Best responses are achieved in patients who have had a prior remission of at least 1 year.

Session topic: E-poster
Keyword(s): Gemcitabine, High-grade non-Hodgkins-lymphoma
Type: Eposter Presentation
Background
Relapsed/refractory aggressive lymphoma remains challenging to treat. There is no standard salvage chemotherapy regimen. Studies suggest that the outpatient combination of Gemcitabine, Oxaliplatin (Gem-Ox) with Rituximab (R) achieves similar response rates (RR) with less toxicity than intensive inpatient regimens1.
Aims
To review outcomes for patients with refractory/relapsed aggressive lymphoma treated with Gem/Ox+/-R.
Methods
This retrospective study analysed clinical data on patients with relapsed/refractory aggressive lymphoma treated with Gem/Ox with or without R between 2010 and 2015 at 3 teaching hospitals in London, UK. The treatment schedule was Gemcitabine 1000mg/m2 and Oxaliplatin 100mg/m2 +/- Rituximab 375 mg/m2 on day 1 and repeated every 15 days for up to 8 cycles.
Results
44 patients, 26 male, with a median age of 66 years (range 22-83) were included. 30 (68%) patients had DLBCL; 27 had received prior R. 6 had T-cell lymphoma, 3 Hodgkin lymphoma and 5 had high grade transformation of low grade B-cell lymphoma. The median number of prior treatments was 1 (range 1-6). 7 patients had received an autologous transplant, 1 of whom had also received an allogeneic transplant. Remission duration (RD) to the prior treatment regimen was <12 months in 26 (59%) patients. 26 (75%) patients received R with Gem-Ox. The median number of cycles received was 4 (range 1-6). Response was assessed by CT scan in 19 PET/CT in 19, MRI in 2 and by clinical evaluation in 4. The overall response was 43% (n=19) with a complete response (CR) of 29% (n=13). CR was achieved in 11/18 (61%) patients with a prior RD ≥12 months compared to 2/26 (8%) patients whose prior RD was <12 months (p=0.0002). 27/44 (61%) patients were transplant eligible, 7 of whom had received a prior autologous transplant. The overall response in transplant eligible patients was 44 % (n=12) 8/27 (30%) went on to receive a stem cell transplant; 4 autologous and 4 allogeneic transplants. At a median follow up of 6 months (range 0.5-33), the median overall survival (OS) is 8 months. For the 19 responding patients the median RD has not been reached. Median OS was significantly better in patients who had had a ≥12 month response to their prior treatment regimen; 13 vs 4.5 months, p=0.02 (Fig 1). Toxicity data were available for 36 patients. 17(47%) patients experienced grade 3/4 haematological toxicity. 8 (22%) had at least one febrile episode requiring hospitalisation. There were 4 (11%) grade 3/4 non-haematological toxicities, including diarrhoea, vomiting, peripheral neuropathy and bowel perforation. Dose reductions were required in 5 patients. 33 (75%) patients have died, 26 (60%) from lymphoma. Other causes of death include 1 old age, 2 post transplant graft vs host disease, 1 treatment-related sepsis 3 unknown.
Conclusion
In this study of ‘real world’ patients, Gem-Ox+/-R is a well-tolerated, outpatient regimen achieving good response rates in patients with chemo-responsive disease. Responses are comparable to more toxic, inpatient, platinum-based regimens. It can also successfully bridge patients to stem cell transplantation. Best responses are achieved in patients who have had a prior remission of at least 1 year.

Session topic: E-poster
Keyword(s): Gemcitabine, High-grade non-Hodgkins-lymphoma
{{ help_message }}
{{filter}}


