FEASIBILITY AND EFFICACY OF DHAOX REGIMEN AS SALVAGE AND MOBILIZING THERAPY IN RELAPSED /REFRACTORY LYMPHOMAS.
(Abstract release date: 05/19/16)
EHA Library. Coviello E. 06/09/16; 132521; E972

Dr. Elisa Coviello
Contributions
Contributions
Abstract
Abstract: E972
Type: Eposter Presentation
Background
As already known, cisplatinum containing regimens for the management of relapsed-refractory lymphomas are complicated by different adverse events, mainly renal impairment. Since 2004 we used oxaliplatinum instead of cisplatinum, in association with cytarabine and dexamethasone (DHAOX).
Aims
The aim of the present study was to perform a retrospective analysis on feasibility and efficacy of this salvage regimen in patients with R/R lymphomas.
Methods
We retrospectively analyzed the outcome of 83 patients with R/R lymphomas who received DHAOX regimen from October 2004 to January 2016. The median age was 59 (22-79) and the median follow up is 56 months. Our series includes 41 patients with Diffuse Large B cell Lymphoma (DLBCL, 49%), 10 with Hodgkin Lymphoma (HL, 12%), 13 with Mantle Cell Lymphoma (MCL,16%), 15 with Indolent Lymphomas (18%), 4 with Peripheral T cell Lymphoma (5%). DHAOX schedule contains dexamethasone (40 mg/die on days 1-4), oxaliplatinum (130 mg/sqm on day 1) and cytarabine (2 g/sqm bid on day 2). In the majority of patients DHAOX was used as second line therapy. All patients received prophylactic G-CSF to reduce neutropenia. We analyzed overall survival (OS) and relapse free survival (RFS) in the whole cohort and then in DLBCL and HL patients. We evaluated haematological and non haematological adverse events (AEs).
Results
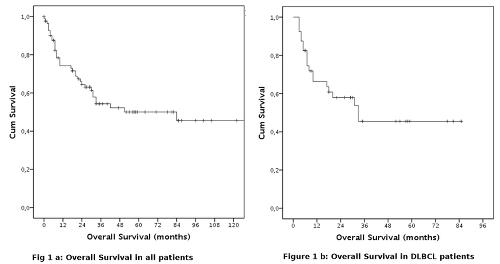
Non haematological AEs of grade > 2 were observed in 27 patients (33%). The most frequent were: oral mucositis and other gastrointestinal events (6 patients, 8%), FUO (17 patients, 21%), sepsis (3 patients, 4%), paresthesias (5 patients, 6%), and other different AEs in 4 patients (gout, atrial fibrillation, hypocalcemia and pleural effusion). 13 patients (16%) needed transfusional support. No patients experienced renal impairment.In 39 patients DHAOX regimen was utilized as mobilizing therapy to collect CD34+ cells for autologous stem cell transplantation (ASCT). In 32/40 patients (83%) more than 4x106 CD34+ cells/kg were collected following G-CSF stimulation (5 mcg/Kg/die from day 5), in 1 patient associated with plerixafor.Median OS was 84 months. The projected OS at 12, 24, 36 months was 74.5%, 64.3% and 54.5%, respectively.Forty-one patients with DLBCL have been treated (median age 55 yy, range 22-79), 33 in second line (81%), 8 in third or subsequent line (19%). CR was obtained in 22 cases (60%), PR in 4 cases (11%). Eleven patients did not respond (29%). ORR was 71%. The median OS was 33 months and the projected OS at 12, 24 and 36 months was 66.3%, 57.9%, 45.5% respectively.Ten patients with HL were treated (median age 44, range 28-74), 7 in second or third line (70%), 3 in fourth or subsequent lines (30%). CR and PR were obtained in 3 (30%) and 1 patients (10%), respectively. ORR was 40%. Considering only patients in second or third line, the ORR was 29%. Stem cell harvest was attempted in 2 patients, reaching the stem cell goal in both cases. The median OS was 84 months. The projected OS at 12, 24 e 36 months was 88.9%, 77.8%, 66.7% respectively.
Conclusion
DHAOX regimen was generally well tolerated, with acceptable toxicity profile and no documented significant renal impairment. By reducing days of hospitalization and the need of supportive care, we obtained a significant saving of economic resources despite the higher cost of oxaliplatinum. Stem cell mobilization with DHAOX resulted feasible and a successful apheresis was achieved in most patients. In conclusion, DHAOX regimen showed a good efficacy and a safe profile in the setting of relapsed/refractory non Hodgkin Lymphomas, mainly DLBCL; on the contrary, we experienced poor results in relapsed/refractory HL, suggesting that other schedules might be better in these patients.
Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, NHL, Oxaliplatin, Relapsed lymphoma
Type: Eposter Presentation
Background
As already known, cisplatinum containing regimens for the management of relapsed-refractory lymphomas are complicated by different adverse events, mainly renal impairment. Since 2004 we used oxaliplatinum instead of cisplatinum, in association with cytarabine and dexamethasone (DHAOX).
Aims
The aim of the present study was to perform a retrospective analysis on feasibility and efficacy of this salvage regimen in patients with R/R lymphomas.
Methods
We retrospectively analyzed the outcome of 83 patients with R/R lymphomas who received DHAOX regimen from October 2004 to January 2016. The median age was 59 (22-79) and the median follow up is 56 months. Our series includes 41 patients with Diffuse Large B cell Lymphoma (DLBCL, 49%), 10 with Hodgkin Lymphoma (HL, 12%), 13 with Mantle Cell Lymphoma (MCL,16%), 15 with Indolent Lymphomas (18%), 4 with Peripheral T cell Lymphoma (5%). DHAOX schedule contains dexamethasone (40 mg/die on days 1-4), oxaliplatinum (130 mg/sqm on day 1) and cytarabine (2 g/sqm bid on day 2). In the majority of patients DHAOX was used as second line therapy. All patients received prophylactic G-CSF to reduce neutropenia. We analyzed overall survival (OS) and relapse free survival (RFS) in the whole cohort and then in DLBCL and HL patients. We evaluated haematological and non haematological adverse events (AEs).
Results
Non haematological AEs of grade > 2 were observed in 27 patients (33%). The most frequent were: oral mucositis and other gastrointestinal events (6 patients, 8%), FUO (17 patients, 21%), sepsis (3 patients, 4%), paresthesias (5 patients, 6%), and other different AEs in 4 patients (gout, atrial fibrillation, hypocalcemia and pleural effusion). 13 patients (16%) needed transfusional support. No patients experienced renal impairment.In 39 patients DHAOX regimen was utilized as mobilizing therapy to collect CD34+ cells for autologous stem cell transplantation (ASCT). In 32/40 patients (83%) more than 4x106 CD34+ cells/kg were collected following G-CSF stimulation (5 mcg/Kg/die from day 5), in 1 patient associated with plerixafor.Median OS was 84 months. The projected OS at 12, 24, 36 months was 74.5%, 64.3% and 54.5%, respectively.Forty-one patients with DLBCL have been treated (median age 55 yy, range 22-79), 33 in second line (81%), 8 in third or subsequent line (19%). CR was obtained in 22 cases (60%), PR in 4 cases (11%). Eleven patients did not respond (29%). ORR was 71%. The median OS was 33 months and the projected OS at 12, 24 and 36 months was 66.3%, 57.9%, 45.5% respectively.Ten patients with HL were treated (median age 44, range 28-74), 7 in second or third line (70%), 3 in fourth or subsequent lines (30%). CR and PR were obtained in 3 (30%) and 1 patients (10%), respectively. ORR was 40%. Considering only patients in second or third line, the ORR was 29%. Stem cell harvest was attempted in 2 patients, reaching the stem cell goal in both cases. The median OS was 84 months. The projected OS at 12, 24 e 36 months was 88.9%, 77.8%, 66.7% respectively.
Conclusion
DHAOX regimen was generally well tolerated, with acceptable toxicity profile and no documented significant renal impairment. By reducing days of hospitalization and the need of supportive care, we obtained a significant saving of economic resources despite the higher cost of oxaliplatinum. Stem cell mobilization with DHAOX resulted feasible and a successful apheresis was achieved in most patients. In conclusion, DHAOX regimen showed a good efficacy and a safe profile in the setting of relapsed/refractory non Hodgkin Lymphomas, mainly DLBCL; on the contrary, we experienced poor results in relapsed/refractory HL, suggesting that other schedules might be better in these patients.

Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, NHL, Oxaliplatin, Relapsed lymphoma
Abstract: E972
Type: Eposter Presentation
Background
As already known, cisplatinum containing regimens for the management of relapsed-refractory lymphomas are complicated by different adverse events, mainly renal impairment. Since 2004 we used oxaliplatinum instead of cisplatinum, in association with cytarabine and dexamethasone (DHAOX).
Aims
The aim of the present study was to perform a retrospective analysis on feasibility and efficacy of this salvage regimen in patients with R/R lymphomas.
Methods
We retrospectively analyzed the outcome of 83 patients with R/R lymphomas who received DHAOX regimen from October 2004 to January 2016. The median age was 59 (22-79) and the median follow up is 56 months. Our series includes 41 patients with Diffuse Large B cell Lymphoma (DLBCL, 49%), 10 with Hodgkin Lymphoma (HL, 12%), 13 with Mantle Cell Lymphoma (MCL,16%), 15 with Indolent Lymphomas (18%), 4 with Peripheral T cell Lymphoma (5%). DHAOX schedule contains dexamethasone (40 mg/die on days 1-4), oxaliplatinum (130 mg/sqm on day 1) and cytarabine (2 g/sqm bid on day 2). In the majority of patients DHAOX was used as second line therapy. All patients received prophylactic G-CSF to reduce neutropenia. We analyzed overall survival (OS) and relapse free survival (RFS) in the whole cohort and then in DLBCL and HL patients. We evaluated haematological and non haematological adverse events (AEs).
Results
Non haematological AEs of grade > 2 were observed in 27 patients (33%). The most frequent were: oral mucositis and other gastrointestinal events (6 patients, 8%), FUO (17 patients, 21%), sepsis (3 patients, 4%), paresthesias (5 patients, 6%), and other different AEs in 4 patients (gout, atrial fibrillation, hypocalcemia and pleural effusion). 13 patients (16%) needed transfusional support. No patients experienced renal impairment.In 39 patients DHAOX regimen was utilized as mobilizing therapy to collect CD34+ cells for autologous stem cell transplantation (ASCT). In 32/40 patients (83%) more than 4x106 CD34+ cells/kg were collected following G-CSF stimulation (5 mcg/Kg/die from day 5), in 1 patient associated with plerixafor.Median OS was 84 months. The projected OS at 12, 24, 36 months was 74.5%, 64.3% and 54.5%, respectively.Forty-one patients with DLBCL have been treated (median age 55 yy, range 22-79), 33 in second line (81%), 8 in third or subsequent line (19%). CR was obtained in 22 cases (60%), PR in 4 cases (11%). Eleven patients did not respond (29%). ORR was 71%. The median OS was 33 months and the projected OS at 12, 24 and 36 months was 66.3%, 57.9%, 45.5% respectively.Ten patients with HL were treated (median age 44, range 28-74), 7 in second or third line (70%), 3 in fourth or subsequent lines (30%). CR and PR were obtained in 3 (30%) and 1 patients (10%), respectively. ORR was 40%. Considering only patients in second or third line, the ORR was 29%. Stem cell harvest was attempted in 2 patients, reaching the stem cell goal in both cases. The median OS was 84 months. The projected OS at 12, 24 e 36 months was 88.9%, 77.8%, 66.7% respectively.
Conclusion
DHAOX regimen was generally well tolerated, with acceptable toxicity profile and no documented significant renal impairment. By reducing days of hospitalization and the need of supportive care, we obtained a significant saving of economic resources despite the higher cost of oxaliplatinum. Stem cell mobilization with DHAOX resulted feasible and a successful apheresis was achieved in most patients. In conclusion, DHAOX regimen showed a good efficacy and a safe profile in the setting of relapsed/refractory non Hodgkin Lymphomas, mainly DLBCL; on the contrary, we experienced poor results in relapsed/refractory HL, suggesting that other schedules might be better in these patients.

Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, NHL, Oxaliplatin, Relapsed lymphoma
Type: Eposter Presentation
Background
As already known, cisplatinum containing regimens for the management of relapsed-refractory lymphomas are complicated by different adverse events, mainly renal impairment. Since 2004 we used oxaliplatinum instead of cisplatinum, in association with cytarabine and dexamethasone (DHAOX).
Aims
The aim of the present study was to perform a retrospective analysis on feasibility and efficacy of this salvage regimen in patients with R/R lymphomas.
Methods
We retrospectively analyzed the outcome of 83 patients with R/R lymphomas who received DHAOX regimen from October 2004 to January 2016. The median age was 59 (22-79) and the median follow up is 56 months. Our series includes 41 patients with Diffuse Large B cell Lymphoma (DLBCL, 49%), 10 with Hodgkin Lymphoma (HL, 12%), 13 with Mantle Cell Lymphoma (MCL,16%), 15 with Indolent Lymphomas (18%), 4 with Peripheral T cell Lymphoma (5%). DHAOX schedule contains dexamethasone (40 mg/die on days 1-4), oxaliplatinum (130 mg/sqm on day 1) and cytarabine (2 g/sqm bid on day 2). In the majority of patients DHAOX was used as second line therapy. All patients received prophylactic G-CSF to reduce neutropenia. We analyzed overall survival (OS) and relapse free survival (RFS) in the whole cohort and then in DLBCL and HL patients. We evaluated haematological and non haematological adverse events (AEs).
Results
Non haematological AEs of grade > 2 were observed in 27 patients (33%). The most frequent were: oral mucositis and other gastrointestinal events (6 patients, 8%), FUO (17 patients, 21%), sepsis (3 patients, 4%), paresthesias (5 patients, 6%), and other different AEs in 4 patients (gout, atrial fibrillation, hypocalcemia and pleural effusion). 13 patients (16%) needed transfusional support. No patients experienced renal impairment.In 39 patients DHAOX regimen was utilized as mobilizing therapy to collect CD34+ cells for autologous stem cell transplantation (ASCT). In 32/40 patients (83%) more than 4x106 CD34+ cells/kg were collected following G-CSF stimulation (5 mcg/Kg/die from day 5), in 1 patient associated with plerixafor.Median OS was 84 months. The projected OS at 12, 24, 36 months was 74.5%, 64.3% and 54.5%, respectively.Forty-one patients with DLBCL have been treated (median age 55 yy, range 22-79), 33 in second line (81%), 8 in third or subsequent line (19%). CR was obtained in 22 cases (60%), PR in 4 cases (11%). Eleven patients did not respond (29%). ORR was 71%. The median OS was 33 months and the projected OS at 12, 24 and 36 months was 66.3%, 57.9%, 45.5% respectively.Ten patients with HL were treated (median age 44, range 28-74), 7 in second or third line (70%), 3 in fourth or subsequent lines (30%). CR and PR were obtained in 3 (30%) and 1 patients (10%), respectively. ORR was 40%. Considering only patients in second or third line, the ORR was 29%. Stem cell harvest was attempted in 2 patients, reaching the stem cell goal in both cases. The median OS was 84 months. The projected OS at 12, 24 e 36 months was 88.9%, 77.8%, 66.7% respectively.
Conclusion
DHAOX regimen was generally well tolerated, with acceptable toxicity profile and no documented significant renal impairment. By reducing days of hospitalization and the need of supportive care, we obtained a significant saving of economic resources despite the higher cost of oxaliplatinum. Stem cell mobilization with DHAOX resulted feasible and a successful apheresis was achieved in most patients. In conclusion, DHAOX regimen showed a good efficacy and a safe profile in the setting of relapsed/refractory non Hodgkin Lymphomas, mainly DLBCL; on the contrary, we experienced poor results in relapsed/refractory HL, suggesting that other schedules might be better in these patients.

Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, NHL, Oxaliplatin, Relapsed lymphoma
{{ help_message }}
{{filter}}


