RITUXIMAB AND HIGH DOSE CYTARABINE WITH AUTOLOGOUS TRANSPLANTATION FOR MANTLE CELL LYMPHOMA
(Abstract release date: 05/19/16)
EHA Library. Burrows S. 06/09/16; 132519; E970

Samuel Howard Burrows
Contributions
Contributions
Abstract
Abstract: E970
Type: Eposter Presentation
Background
Mantle cell lymphoma (MCL) is an aggressive and incurable form of non-Hodgkin lymphoma (NHL), typically presenting at a late stage, often with extra nodal involvement, and with a preponderance for older males. Front-line therapy incorporating rituximab and high-dose cytarabine (R-HDAC), often in combination with other drugs or alternating with another regimen, followed by autograft in first response is standard of care for younger, fitter patients. Reported overall response rates (ORR) and progression free survival (PFS) are typically 70-90% and 50-60% respectively.
Aims
To explore the tolerability and efficacy of rituximab and single-agent R-HDAC prior to autologous stem-cell transplant in patients with untreated MCL.
Methods
We studied 45 unselected, transplant eligible patients with MCL treated upfront with R-HDAC alone across four UK centres between February 2010 and August 2015. The median age was 60, the majority were male (84%), and the majority had advanced disease (91% Stage III/IV). None had central nervous system (CNS) involvement at presentation. All patients received at least one cycle of R-HDAC. The median number of cycles of R-HDAC given was 5, and 89% received at least three cycles. Only two patients stopped R-HDAC therapy early due to toxicity; one due to PJP pneumonia after the first cycle who was stepped down to Bendamustine based treatment, and one due to severe sepsis after the second cycle who stopped therapy at this point having already achieved a partial response. One further patient completed five cycles of R-HDAC but was felt unfit for autograft at that point.
Results
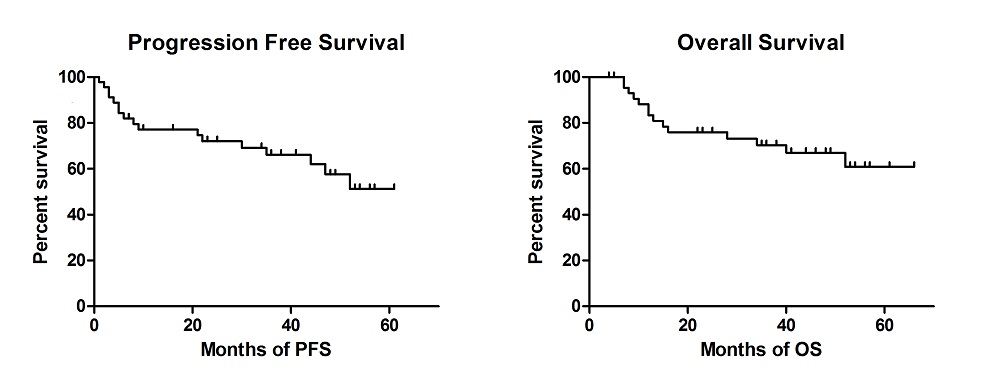
Nine patients (20%) progressed on R-HDAC, of whom only three (33%) achieved a remission with second line therapy, two of whom subsequently died of progressive disease. In total 31 patients (69%) have now undergone transplantation; 28 (62%) received an autograft (the majority BEAM conditioned), two had an allogeneic transplant and one a syngeneic transplant. Two patients are currently completing R-HDAC treatment and will undergo BEAM autograft thereafter. Transplant related mortality was within expected limits, with one patient dying as a result of sepsis due to BEAM autograft toxicity. Median duration of follow up of the entire cohort was 38 months. Overall response rate for R-HDAC with or without transplantation was 35/45 (78%), with most patients achieving a complete remission (CR) (n=30, 67%). Median OS and PFS were not reached. The OS at 4yrs is 78% (18/23); and PFS at 4yrs is 61% (14/23). Eight patients relapsed after achieving CR1 with HDAC +/- transplantation, five (63%) have subsequently achieved a second remission. The most commonly employed strategies amongst patients who required subsequent treatment were Ibrutinib (47%), Bortezomib (35%), CHOP (29%) and Bendamustine (17%) based therapies. CNS involvement was seen at relapse/progression in four cases.
Conclusion
This study confirms that induction with R-HDAC, consolidated with stem cell transplantation where appropriate, is a safe and effective upfront treatment strategy in MCL, and compares favourably with other induction regimens in terms of toxicity and duration of response. By avoiding the use of other agents as part of upfront treatment excess toxicity is avoided. Using R-HDAC upfront also leaves a greater range of therapies available for use at subsequent relapse, with good remission rates after second line therapy shown in this study. However, direct comparison with other upfront treatment strategies has not been made, and a prospective randomised controlled trial is needed.
Session topic: E-poster
Type: Eposter Presentation
Background
Mantle cell lymphoma (MCL) is an aggressive and incurable form of non-Hodgkin lymphoma (NHL), typically presenting at a late stage, often with extra nodal involvement, and with a preponderance for older males. Front-line therapy incorporating rituximab and high-dose cytarabine (R-HDAC), often in combination with other drugs or alternating with another regimen, followed by autograft in first response is standard of care for younger, fitter patients. Reported overall response rates (ORR) and progression free survival (PFS) are typically 70-90% and 50-60% respectively.
Aims
To explore the tolerability and efficacy of rituximab and single-agent R-HDAC prior to autologous stem-cell transplant in patients with untreated MCL.
Methods
We studied 45 unselected, transplant eligible patients with MCL treated upfront with R-HDAC alone across four UK centres between February 2010 and August 2015. The median age was 60, the majority were male (84%), and the majority had advanced disease (91% Stage III/IV). None had central nervous system (CNS) involvement at presentation. All patients received at least one cycle of R-HDAC. The median number of cycles of R-HDAC given was 5, and 89% received at least three cycles. Only two patients stopped R-HDAC therapy early due to toxicity; one due to PJP pneumonia after the first cycle who was stepped down to Bendamustine based treatment, and one due to severe sepsis after the second cycle who stopped therapy at this point having already achieved a partial response. One further patient completed five cycles of R-HDAC but was felt unfit for autograft at that point.
Results
Nine patients (20%) progressed on R-HDAC, of whom only three (33%) achieved a remission with second line therapy, two of whom subsequently died of progressive disease. In total 31 patients (69%) have now undergone transplantation; 28 (62%) received an autograft (the majority BEAM conditioned), two had an allogeneic transplant and one a syngeneic transplant. Two patients are currently completing R-HDAC treatment and will undergo BEAM autograft thereafter. Transplant related mortality was within expected limits, with one patient dying as a result of sepsis due to BEAM autograft toxicity. Median duration of follow up of the entire cohort was 38 months. Overall response rate for R-HDAC with or without transplantation was 35/45 (78%), with most patients achieving a complete remission (CR) (n=30, 67%). Median OS and PFS were not reached. The OS at 4yrs is 78% (18/23); and PFS at 4yrs is 61% (14/23). Eight patients relapsed after achieving CR1 with HDAC +/- transplantation, five (63%) have subsequently achieved a second remission. The most commonly employed strategies amongst patients who required subsequent treatment were Ibrutinib (47%), Bortezomib (35%), CHOP (29%) and Bendamustine (17%) based therapies. CNS involvement was seen at relapse/progression in four cases.
Conclusion
This study confirms that induction with R-HDAC, consolidated with stem cell transplantation where appropriate, is a safe and effective upfront treatment strategy in MCL, and compares favourably with other induction regimens in terms of toxicity and duration of response. By avoiding the use of other agents as part of upfront treatment excess toxicity is avoided. Using R-HDAC upfront also leaves a greater range of therapies available for use at subsequent relapse, with good remission rates after second line therapy shown in this study. However, direct comparison with other upfront treatment strategies has not been made, and a prospective randomised controlled trial is needed.

Session topic: E-poster
Abstract: E970
Type: Eposter Presentation
Background
Mantle cell lymphoma (MCL) is an aggressive and incurable form of non-Hodgkin lymphoma (NHL), typically presenting at a late stage, often with extra nodal involvement, and with a preponderance for older males. Front-line therapy incorporating rituximab and high-dose cytarabine (R-HDAC), often in combination with other drugs or alternating with another regimen, followed by autograft in first response is standard of care for younger, fitter patients. Reported overall response rates (ORR) and progression free survival (PFS) are typically 70-90% and 50-60% respectively.
Aims
To explore the tolerability and efficacy of rituximab and single-agent R-HDAC prior to autologous stem-cell transplant in patients with untreated MCL.
Methods
We studied 45 unselected, transplant eligible patients with MCL treated upfront with R-HDAC alone across four UK centres between February 2010 and August 2015. The median age was 60, the majority were male (84%), and the majority had advanced disease (91% Stage III/IV). None had central nervous system (CNS) involvement at presentation. All patients received at least one cycle of R-HDAC. The median number of cycles of R-HDAC given was 5, and 89% received at least three cycles. Only two patients stopped R-HDAC therapy early due to toxicity; one due to PJP pneumonia after the first cycle who was stepped down to Bendamustine based treatment, and one due to severe sepsis after the second cycle who stopped therapy at this point having already achieved a partial response. One further patient completed five cycles of R-HDAC but was felt unfit for autograft at that point.
Results
Nine patients (20%) progressed on R-HDAC, of whom only three (33%) achieved a remission with second line therapy, two of whom subsequently died of progressive disease. In total 31 patients (69%) have now undergone transplantation; 28 (62%) received an autograft (the majority BEAM conditioned), two had an allogeneic transplant and one a syngeneic transplant. Two patients are currently completing R-HDAC treatment and will undergo BEAM autograft thereafter. Transplant related mortality was within expected limits, with one patient dying as a result of sepsis due to BEAM autograft toxicity. Median duration of follow up of the entire cohort was 38 months. Overall response rate for R-HDAC with or without transplantation was 35/45 (78%), with most patients achieving a complete remission (CR) (n=30, 67%). Median OS and PFS were not reached. The OS at 4yrs is 78% (18/23); and PFS at 4yrs is 61% (14/23). Eight patients relapsed after achieving CR1 with HDAC +/- transplantation, five (63%) have subsequently achieved a second remission. The most commonly employed strategies amongst patients who required subsequent treatment were Ibrutinib (47%), Bortezomib (35%), CHOP (29%) and Bendamustine (17%) based therapies. CNS involvement was seen at relapse/progression in four cases.
Conclusion
This study confirms that induction with R-HDAC, consolidated with stem cell transplantation where appropriate, is a safe and effective upfront treatment strategy in MCL, and compares favourably with other induction regimens in terms of toxicity and duration of response. By avoiding the use of other agents as part of upfront treatment excess toxicity is avoided. Using R-HDAC upfront also leaves a greater range of therapies available for use at subsequent relapse, with good remission rates after second line therapy shown in this study. However, direct comparison with other upfront treatment strategies has not been made, and a prospective randomised controlled trial is needed.

Session topic: E-poster
Type: Eposter Presentation
Background
Mantle cell lymphoma (MCL) is an aggressive and incurable form of non-Hodgkin lymphoma (NHL), typically presenting at a late stage, often with extra nodal involvement, and with a preponderance for older males. Front-line therapy incorporating rituximab and high-dose cytarabine (R-HDAC), often in combination with other drugs or alternating with another regimen, followed by autograft in first response is standard of care for younger, fitter patients. Reported overall response rates (ORR) and progression free survival (PFS) are typically 70-90% and 50-60% respectively.
Aims
To explore the tolerability and efficacy of rituximab and single-agent R-HDAC prior to autologous stem-cell transplant in patients with untreated MCL.
Methods
We studied 45 unselected, transplant eligible patients with MCL treated upfront with R-HDAC alone across four UK centres between February 2010 and August 2015. The median age was 60, the majority were male (84%), and the majority had advanced disease (91% Stage III/IV). None had central nervous system (CNS) involvement at presentation. All patients received at least one cycle of R-HDAC. The median number of cycles of R-HDAC given was 5, and 89% received at least three cycles. Only two patients stopped R-HDAC therapy early due to toxicity; one due to PJP pneumonia after the first cycle who was stepped down to Bendamustine based treatment, and one due to severe sepsis after the second cycle who stopped therapy at this point having already achieved a partial response. One further patient completed five cycles of R-HDAC but was felt unfit for autograft at that point.
Results
Nine patients (20%) progressed on R-HDAC, of whom only three (33%) achieved a remission with second line therapy, two of whom subsequently died of progressive disease. In total 31 patients (69%) have now undergone transplantation; 28 (62%) received an autograft (the majority BEAM conditioned), two had an allogeneic transplant and one a syngeneic transplant. Two patients are currently completing R-HDAC treatment and will undergo BEAM autograft thereafter. Transplant related mortality was within expected limits, with one patient dying as a result of sepsis due to BEAM autograft toxicity. Median duration of follow up of the entire cohort was 38 months. Overall response rate for R-HDAC with or without transplantation was 35/45 (78%), with most patients achieving a complete remission (CR) (n=30, 67%). Median OS and PFS were not reached. The OS at 4yrs is 78% (18/23); and PFS at 4yrs is 61% (14/23). Eight patients relapsed after achieving CR1 with HDAC +/- transplantation, five (63%) have subsequently achieved a second remission. The most commonly employed strategies amongst patients who required subsequent treatment were Ibrutinib (47%), Bortezomib (35%), CHOP (29%) and Bendamustine (17%) based therapies. CNS involvement was seen at relapse/progression in four cases.
Conclusion
This study confirms that induction with R-HDAC, consolidated with stem cell transplantation where appropriate, is a safe and effective upfront treatment strategy in MCL, and compares favourably with other induction regimens in terms of toxicity and duration of response. By avoiding the use of other agents as part of upfront treatment excess toxicity is avoided. Using R-HDAC upfront also leaves a greater range of therapies available for use at subsequent relapse, with good remission rates after second line therapy shown in this study. However, direct comparison with other upfront treatment strategies has not been made, and a prospective randomised controlled trial is needed.

Session topic: E-poster
{{ help_message }}
{{filter}}


