EXTRACORPOREAL PHOTOPHERESIS: SAFE AND EFFECTIVE FOR THE TREATMENT OF MYCOSIS FUNGOIDES
(Abstract release date: 05/19/16)
EHA Library. Atilla E. 06/09/16; 132506; E957

Dr. Erden Atilla
Contributions
Contributions
Abstract
Abstract: E957
Type: Eposter Presentation
Background
Primary cutaneous T-cell lymphomas (CTCL) are second most common extranodal non-Hodgkin lymphoma with an yearly incidence of 0.45 per 100,000 person. The treatment of mycosis fungoides (MF), the most frequent CTCL, is determined by disease extent, prognostic factors and patient characteristics. Extracorporeal Photopheresis (ECP) was approved by the US Food and Drug Administiration for the palliative treatment of MF since 1988.
Aims
The aim of this study is to analyze the clinical activity, toxicities, response and outcome rates of ECP; compare with combination therapies in the treatment of patients with MF.
Methods
We retrospectively included 50 MF patients who have diagnosed at our center. ECP was given empirically in cycles of 2 consecutive days in every 2 to 4 weeks at any time during their follow-up.
Results
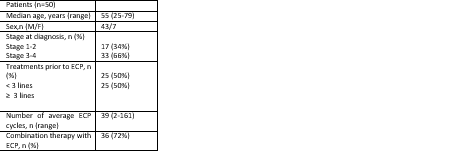
The patient characteristics is shown in table. Treatments prior to ECP were; topical retinoids (bexarotene), topical corticosteroids, phototherapy (PUVA), Narrowband ultraviolet B light (NBUVB), Interferons, Metotrexate (MTX), CHOP (cyclophosphamide, daunorubicin, vincristine, prednisolone). ECP is combined with gemcitabine, PUVA, MTX, Bexaroten, IFN or Vorinostat up to patient characteristics. Overall response rate (ORR) was 42% (21/50) with median time to response 11 months (range, 3-48 months). 15 of the responded patients (71%) were in stage III and IV at diagnosis. 10 of the responders (48%) had 3 or over treatment lines prior to ECP. 29 patient had not responded (58%) and 3 patients underwent (6%) allogeneic hematopoietic stem cell transplantation during follow-up. 8 patients (16%) had adverse events related with ECP; catheter related infection, headache, fever, shivering, nausea. Overall survival (OS) rate for 50 patients were 72 months (range, 3-211). The number of cycles in ECP responder patients were 32, and 44 for non-responders. Stage 3 and 4 patients had received average of 31 cycles compared to 55 cycles in stage 1 and 2 patients (P=0.006). The increased number of ECP is not correlated with the response (P=0.203). There were no statistically difference in OS in early stage vs late stage patients (77 vs 69 months, P=0.077). Time to response in early stage disease were 14 months and 8 months with late stage disease (P=0.267). Combined treatment with ECP statistically had improved OS (84 months vs 62 months, P=0.005).
Conclusion
The overall survival has improved in combined treatment with ECP patients. ECP may be a preferred treatment alternative due to low risk of adverse events. There is a conflicting data on treatment schedule and continuation. Prospective controlled clinical trials which are conducted on same ECP protocol and duration will better document the efficacy of this therapeutic modality.
Session topic: E-poster
Keyword(s): Extracorporeal photopheresis, Mycosis fungoides
Type: Eposter Presentation
Background
Primary cutaneous T-cell lymphomas (CTCL) are second most common extranodal non-Hodgkin lymphoma with an yearly incidence of 0.45 per 100,000 person. The treatment of mycosis fungoides (MF), the most frequent CTCL, is determined by disease extent, prognostic factors and patient characteristics. Extracorporeal Photopheresis (ECP) was approved by the US Food and Drug Administiration for the palliative treatment of MF since 1988.
Aims
The aim of this study is to analyze the clinical activity, toxicities, response and outcome rates of ECP; compare with combination therapies in the treatment of patients with MF.
Methods
We retrospectively included 50 MF patients who have diagnosed at our center. ECP was given empirically in cycles of 2 consecutive days in every 2 to 4 weeks at any time during their follow-up.
Results
The patient characteristics is shown in table. Treatments prior to ECP were; topical retinoids (bexarotene), topical corticosteroids, phototherapy (PUVA), Narrowband ultraviolet B light (NBUVB), Interferons, Metotrexate (MTX), CHOP (cyclophosphamide, daunorubicin, vincristine, prednisolone). ECP is combined with gemcitabine, PUVA, MTX, Bexaroten, IFN or Vorinostat up to patient characteristics. Overall response rate (ORR) was 42% (21/50) with median time to response 11 months (range, 3-48 months). 15 of the responded patients (71%) were in stage III and IV at diagnosis. 10 of the responders (48%) had 3 or over treatment lines prior to ECP. 29 patient had not responded (58%) and 3 patients underwent (6%) allogeneic hematopoietic stem cell transplantation during follow-up. 8 patients (16%) had adverse events related with ECP; catheter related infection, headache, fever, shivering, nausea. Overall survival (OS) rate for 50 patients were 72 months (range, 3-211). The number of cycles in ECP responder patients were 32, and 44 for non-responders. Stage 3 and 4 patients had received average of 31 cycles compared to 55 cycles in stage 1 and 2 patients (P=0.006). The increased number of ECP is not correlated with the response (P=0.203). There were no statistically difference in OS in early stage vs late stage patients (77 vs 69 months, P=0.077). Time to response in early stage disease were 14 months and 8 months with late stage disease (P=0.267). Combined treatment with ECP statistically had improved OS (84 months vs 62 months, P=0.005).
Conclusion
The overall survival has improved in combined treatment with ECP patients. ECP may be a preferred treatment alternative due to low risk of adverse events. There is a conflicting data on treatment schedule and continuation. Prospective controlled clinical trials which are conducted on same ECP protocol and duration will better document the efficacy of this therapeutic modality.

Session topic: E-poster
Keyword(s): Extracorporeal photopheresis, Mycosis fungoides
Abstract: E957
Type: Eposter Presentation
Background
Primary cutaneous T-cell lymphomas (CTCL) are second most common extranodal non-Hodgkin lymphoma with an yearly incidence of 0.45 per 100,000 person. The treatment of mycosis fungoides (MF), the most frequent CTCL, is determined by disease extent, prognostic factors and patient characteristics. Extracorporeal Photopheresis (ECP) was approved by the US Food and Drug Administiration for the palliative treatment of MF since 1988.
Aims
The aim of this study is to analyze the clinical activity, toxicities, response and outcome rates of ECP; compare with combination therapies in the treatment of patients with MF.
Methods
We retrospectively included 50 MF patients who have diagnosed at our center. ECP was given empirically in cycles of 2 consecutive days in every 2 to 4 weeks at any time during their follow-up.
Results
The patient characteristics is shown in table. Treatments prior to ECP were; topical retinoids (bexarotene), topical corticosteroids, phototherapy (PUVA), Narrowband ultraviolet B light (NBUVB), Interferons, Metotrexate (MTX), CHOP (cyclophosphamide, daunorubicin, vincristine, prednisolone). ECP is combined with gemcitabine, PUVA, MTX, Bexaroten, IFN or Vorinostat up to patient characteristics. Overall response rate (ORR) was 42% (21/50) with median time to response 11 months (range, 3-48 months). 15 of the responded patients (71%) were in stage III and IV at diagnosis. 10 of the responders (48%) had 3 or over treatment lines prior to ECP. 29 patient had not responded (58%) and 3 patients underwent (6%) allogeneic hematopoietic stem cell transplantation during follow-up. 8 patients (16%) had adverse events related with ECP; catheter related infection, headache, fever, shivering, nausea. Overall survival (OS) rate for 50 patients were 72 months (range, 3-211). The number of cycles in ECP responder patients were 32, and 44 for non-responders. Stage 3 and 4 patients had received average of 31 cycles compared to 55 cycles in stage 1 and 2 patients (P=0.006). The increased number of ECP is not correlated with the response (P=0.203). There were no statistically difference in OS in early stage vs late stage patients (77 vs 69 months, P=0.077). Time to response in early stage disease were 14 months and 8 months with late stage disease (P=0.267). Combined treatment with ECP statistically had improved OS (84 months vs 62 months, P=0.005).
Conclusion
The overall survival has improved in combined treatment with ECP patients. ECP may be a preferred treatment alternative due to low risk of adverse events. There is a conflicting data on treatment schedule and continuation. Prospective controlled clinical trials which are conducted on same ECP protocol and duration will better document the efficacy of this therapeutic modality.

Session topic: E-poster
Keyword(s): Extracorporeal photopheresis, Mycosis fungoides
Type: Eposter Presentation
Background
Primary cutaneous T-cell lymphomas (CTCL) are second most common extranodal non-Hodgkin lymphoma with an yearly incidence of 0.45 per 100,000 person. The treatment of mycosis fungoides (MF), the most frequent CTCL, is determined by disease extent, prognostic factors and patient characteristics. Extracorporeal Photopheresis (ECP) was approved by the US Food and Drug Administiration for the palliative treatment of MF since 1988.
Aims
The aim of this study is to analyze the clinical activity, toxicities, response and outcome rates of ECP; compare with combination therapies in the treatment of patients with MF.
Methods
We retrospectively included 50 MF patients who have diagnosed at our center. ECP was given empirically in cycles of 2 consecutive days in every 2 to 4 weeks at any time during their follow-up.
Results
The patient characteristics is shown in table. Treatments prior to ECP were; topical retinoids (bexarotene), topical corticosteroids, phototherapy (PUVA), Narrowband ultraviolet B light (NBUVB), Interferons, Metotrexate (MTX), CHOP (cyclophosphamide, daunorubicin, vincristine, prednisolone). ECP is combined with gemcitabine, PUVA, MTX, Bexaroten, IFN or Vorinostat up to patient characteristics. Overall response rate (ORR) was 42% (21/50) with median time to response 11 months (range, 3-48 months). 15 of the responded patients (71%) were in stage III and IV at diagnosis. 10 of the responders (48%) had 3 or over treatment lines prior to ECP. 29 patient had not responded (58%) and 3 patients underwent (6%) allogeneic hematopoietic stem cell transplantation during follow-up. 8 patients (16%) had adverse events related with ECP; catheter related infection, headache, fever, shivering, nausea. Overall survival (OS) rate for 50 patients were 72 months (range, 3-211). The number of cycles in ECP responder patients were 32, and 44 for non-responders. Stage 3 and 4 patients had received average of 31 cycles compared to 55 cycles in stage 1 and 2 patients (P=0.006). The increased number of ECP is not correlated with the response (P=0.203). There were no statistically difference in OS in early stage vs late stage patients (77 vs 69 months, P=0.077). Time to response in early stage disease were 14 months and 8 months with late stage disease (P=0.267). Combined treatment with ECP statistically had improved OS (84 months vs 62 months, P=0.005).
Conclusion
The overall survival has improved in combined treatment with ECP patients. ECP may be a preferred treatment alternative due to low risk of adverse events. There is a conflicting data on treatment schedule and continuation. Prospective controlled clinical trials which are conducted on same ECP protocol and duration will better document the efficacy of this therapeutic modality.

Session topic: E-poster
Keyword(s): Extracorporeal photopheresis, Mycosis fungoides
{{ help_message }}
{{filter}}


