HEMOGLOBIN LEVEL IMPROVES THE ABILITY OF THE NATIONAL COMPREHENSIVE CANCER NETWORK INTERNATIONAL PROGNOSTIC INDEX TO PREDICT OUTCOME IN DIFFUSE LARGE B-CELL LYMPHOMA - A POPULATION BASED COHORT STUDY.
(Abstract release date: 05/19/16)
EHA Library. Clausen M. 06/09/16; 132501; E952
Disclosure(s): Research funding recieved from Takeda

Dr. Michael Clausen
Contributions
Contributions
Abstract
Abstract: E952
Type: Eposter Presentation
Background
Prognosis and treatment options for patients with diffuse large B-cell lymphoma (DLBCL) has for the last 20 years been conveyed on the basis of The International Prognostic Index (IPI). Improving IPI by adding biomarkers is, however, an ongoing discipline. The National Comprehensive Cancer Network IPI (NCCN-IPI) adds to the classical IPI factors more refined age and lactate dehydrogenase (LDH) strata and weighs extranodal disease according to the involvement or not of high risk organs such as bone marrow, CNS, liver/GI tract or lung. The NCCN-IPI was derived and validated on two cohorts with a mean age of 57- and 62-years, respectively, i.e. slightly younger than one would expect in a population-based DLBCL cohort. In Denmark, the universal healthcare system is tax funded and free of charge for all inhabitants ensuring a broad coverage of high quality healthcare. For lymphoma, a national group elaborate management guidelines and a prospective collection of patient-related data stored in the group’s database (LYFO).
Aims
i) To validate the NCCN-IPI in a population-based cohort of de-novo DLBCL patients treated with anthracycline-based chemotherapy; (ii) to analyze, in a multivariate model, whether adding information on pre-therapeutic hemoglobin level improves on the ability of the NCCN-IPI to predict outcome.
Methods
All patients were diagnosed with de novo DLBCL between 2000 and 2012 and treated with anthracycline-based chemotherapy with or without rituximab (R). Patients with indolent- or composite lymphoma were excluded, as were patients with primary CNS-lymphoma. Clinical data were obtained from LYFO and biochemical data were retrieved partly from LYFO and from hospital laboratories. Data on vital status were obtained from the Danish Civil Registration System. Patients were followed from the date of biopsy until death or the end of study (December 10, 2015). Overall survival was described using Kaplan-Meier curves according to NCCN-IPI, treatment and hemoglobin values. Univariate and multivariate analysis were performed using Cox proportional hazard model including NCCN-IPI group, anemia and treatment as covariates with mutual adjustment. Anemia was defined as hemoglobin<7.3 mmol/l l for women and <8.3 mmol/l for men. The proportional hazard assumption was evaluated graphically using log-log plots. The study was approved by the Danish Data Protection Agency (no. 1-16-02-562-13).
Results
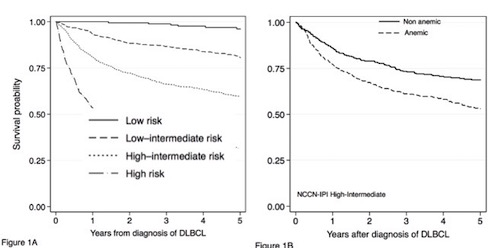
A total of 3654 patients fulfilled the inclusion criteria and had a median follow-up of 1888 days. Median age was 65 years (range 15-98), 2033 (56%) were men, 2081 (57%) had Ann Arbor stage III-IV, and 712 (19%) had a performance score ≥2. The pre-therapeutic LDH ratio (measured vs. max reference value) was >1 in 2225 (61%). Anemia was present in 1784 (49%) patients. The NCCN-IPI separated our cohort in low (8%), low intermediate (35%), high intermediate (39%), and high risk (18%) patients.For R-chemo treated patients, the four NCCN-IPI risk groups (low, low-intermediate, high-intermediate, high) revealed 5-year OS values of 0.96 (CI95%, 0.92-0.98), 0.81 (CI95% 0.77-0.83), 0.60 (CI95% 0.56-0.63), and 0.30 (CI95% 0.26-0.37), respectively (Figure 1A). When hemoglobin level was added to the model it showed a significant impact on OS for both intermediate risk groups. Estimated 5 year overall survival in anemic vs. non anemic patients with high-intermediate risk NCCN-IPI was 0.53 (95%CI 0.48-0.58) and 0.69 (95%CI 0.63-0.73) (Figure 1 B) and for low-intermediate 0.74 (95%CI 0.68-0.79) and 0.84 (95%CI 0.80-0.87). Patients with anemia as defined had a HR of 2.46 (95%CI 2.08-2.90). ). After adjusting for NCCN-IPI factors, the HR remained significant (1.51; 95%CI 1.29-1.83).
Conclusion
In a population-based cohort of de novo DLBCL patients, we found that pre-therapeutic anemia was present in 49% of the patients and had independent prognostic impact also when adjusted for NCCN-IPI risk factors, and stratified for rituximab treatment. The adverse prognostic value of pretherapeutic anemia was independent of gender and age strata. Our patient cohort was older than the ones of the original NCCN-IPI publications (median age 65 yrs vs. 54 yrs), which probably explains the difference in the proportion of high-risk patients (18% in our cohort vs 8% and 14% in the two original NCCN-IPI cohorts).
Session topic: E-poster
Keyword(s): Anemia, Diffuse large B cell lymphoma, Prognostic factor
Type: Eposter Presentation
Background
Prognosis and treatment options for patients with diffuse large B-cell lymphoma (DLBCL) has for the last 20 years been conveyed on the basis of The International Prognostic Index (IPI). Improving IPI by adding biomarkers is, however, an ongoing discipline. The National Comprehensive Cancer Network IPI (NCCN-IPI) adds to the classical IPI factors more refined age and lactate dehydrogenase (LDH) strata and weighs extranodal disease according to the involvement or not of high risk organs such as bone marrow, CNS, liver/GI tract or lung. The NCCN-IPI was derived and validated on two cohorts with a mean age of 57- and 62-years, respectively, i.e. slightly younger than one would expect in a population-based DLBCL cohort. In Denmark, the universal healthcare system is tax funded and free of charge for all inhabitants ensuring a broad coverage of high quality healthcare. For lymphoma, a national group elaborate management guidelines and a prospective collection of patient-related data stored in the group’s database (LYFO).
Aims
i) To validate the NCCN-IPI in a population-based cohort of de-novo DLBCL patients treated with anthracycline-based chemotherapy; (ii) to analyze, in a multivariate model, whether adding information on pre-therapeutic hemoglobin level improves on the ability of the NCCN-IPI to predict outcome.
Methods
All patients were diagnosed with de novo DLBCL between 2000 and 2012 and treated with anthracycline-based chemotherapy with or without rituximab (R). Patients with indolent- or composite lymphoma were excluded, as were patients with primary CNS-lymphoma. Clinical data were obtained from LYFO and biochemical data were retrieved partly from LYFO and from hospital laboratories. Data on vital status were obtained from the Danish Civil Registration System. Patients were followed from the date of biopsy until death or the end of study (December 10, 2015). Overall survival was described using Kaplan-Meier curves according to NCCN-IPI, treatment and hemoglobin values. Univariate and multivariate analysis were performed using Cox proportional hazard model including NCCN-IPI group, anemia and treatment as covariates with mutual adjustment. Anemia was defined as hemoglobin<7.3 mmol/l l for women and <8.3 mmol/l for men. The proportional hazard assumption was evaluated graphically using log-log plots. The study was approved by the Danish Data Protection Agency (no. 1-16-02-562-13).
Results
A total of 3654 patients fulfilled the inclusion criteria and had a median follow-up of 1888 days. Median age was 65 years (range 15-98), 2033 (56%) were men, 2081 (57%) had Ann Arbor stage III-IV, and 712 (19%) had a performance score ≥2. The pre-therapeutic LDH ratio (measured vs. max reference value) was >1 in 2225 (61%). Anemia was present in 1784 (49%) patients. The NCCN-IPI separated our cohort in low (8%), low intermediate (35%), high intermediate (39%), and high risk (18%) patients.For R-chemo treated patients, the four NCCN-IPI risk groups (low, low-intermediate, high-intermediate, high) revealed 5-year OS values of 0.96 (CI95%, 0.92-0.98), 0.81 (CI95% 0.77-0.83), 0.60 (CI95% 0.56-0.63), and 0.30 (CI95% 0.26-0.37), respectively (Figure 1A). When hemoglobin level was added to the model it showed a significant impact on OS for both intermediate risk groups. Estimated 5 year overall survival in anemic vs. non anemic patients with high-intermediate risk NCCN-IPI was 0.53 (95%CI 0.48-0.58) and 0.69 (95%CI 0.63-0.73) (Figure 1 B) and for low-intermediate 0.74 (95%CI 0.68-0.79) and 0.84 (95%CI 0.80-0.87). Patients with anemia as defined had a HR of 2.46 (95%CI 2.08-2.90). ). After adjusting for NCCN-IPI factors, the HR remained significant (1.51; 95%CI 1.29-1.83).
Conclusion
In a population-based cohort of de novo DLBCL patients, we found that pre-therapeutic anemia was present in 49% of the patients and had independent prognostic impact also when adjusted for NCCN-IPI risk factors, and stratified for rituximab treatment. The adverse prognostic value of pretherapeutic anemia was independent of gender and age strata. Our patient cohort was older than the ones of the original NCCN-IPI publications (median age 65 yrs vs. 54 yrs), which probably explains the difference in the proportion of high-risk patients (18% in our cohort vs 8% and 14% in the two original NCCN-IPI cohorts).

Session topic: E-poster
Keyword(s): Anemia, Diffuse large B cell lymphoma, Prognostic factor
Abstract: E952
Type: Eposter Presentation
Background
Prognosis and treatment options for patients with diffuse large B-cell lymphoma (DLBCL) has for the last 20 years been conveyed on the basis of The International Prognostic Index (IPI). Improving IPI by adding biomarkers is, however, an ongoing discipline. The National Comprehensive Cancer Network IPI (NCCN-IPI) adds to the classical IPI factors more refined age and lactate dehydrogenase (LDH) strata and weighs extranodal disease according to the involvement or not of high risk organs such as bone marrow, CNS, liver/GI tract or lung. The NCCN-IPI was derived and validated on two cohorts with a mean age of 57- and 62-years, respectively, i.e. slightly younger than one would expect in a population-based DLBCL cohort. In Denmark, the universal healthcare system is tax funded and free of charge for all inhabitants ensuring a broad coverage of high quality healthcare. For lymphoma, a national group elaborate management guidelines and a prospective collection of patient-related data stored in the group’s database (LYFO).
Aims
i) To validate the NCCN-IPI in a population-based cohort of de-novo DLBCL patients treated with anthracycline-based chemotherapy; (ii) to analyze, in a multivariate model, whether adding information on pre-therapeutic hemoglobin level improves on the ability of the NCCN-IPI to predict outcome.
Methods
All patients were diagnosed with de novo DLBCL between 2000 and 2012 and treated with anthracycline-based chemotherapy with or without rituximab (R). Patients with indolent- or composite lymphoma were excluded, as were patients with primary CNS-lymphoma. Clinical data were obtained from LYFO and biochemical data were retrieved partly from LYFO and from hospital laboratories. Data on vital status were obtained from the Danish Civil Registration System. Patients were followed from the date of biopsy until death or the end of study (December 10, 2015). Overall survival was described using Kaplan-Meier curves according to NCCN-IPI, treatment and hemoglobin values. Univariate and multivariate analysis were performed using Cox proportional hazard model including NCCN-IPI group, anemia and treatment as covariates with mutual adjustment. Anemia was defined as hemoglobin<7.3 mmol/l l for women and <8.3 mmol/l for men. The proportional hazard assumption was evaluated graphically using log-log plots. The study was approved by the Danish Data Protection Agency (no. 1-16-02-562-13).
Results
A total of 3654 patients fulfilled the inclusion criteria and had a median follow-up of 1888 days. Median age was 65 years (range 15-98), 2033 (56%) were men, 2081 (57%) had Ann Arbor stage III-IV, and 712 (19%) had a performance score ≥2. The pre-therapeutic LDH ratio (measured vs. max reference value) was >1 in 2225 (61%). Anemia was present in 1784 (49%) patients. The NCCN-IPI separated our cohort in low (8%), low intermediate (35%), high intermediate (39%), and high risk (18%) patients.For R-chemo treated patients, the four NCCN-IPI risk groups (low, low-intermediate, high-intermediate, high) revealed 5-year OS values of 0.96 (CI95%, 0.92-0.98), 0.81 (CI95% 0.77-0.83), 0.60 (CI95% 0.56-0.63), and 0.30 (CI95% 0.26-0.37), respectively (Figure 1A). When hemoglobin level was added to the model it showed a significant impact on OS for both intermediate risk groups. Estimated 5 year overall survival in anemic vs. non anemic patients with high-intermediate risk NCCN-IPI was 0.53 (95%CI 0.48-0.58) and 0.69 (95%CI 0.63-0.73) (Figure 1 B) and for low-intermediate 0.74 (95%CI 0.68-0.79) and 0.84 (95%CI 0.80-0.87). Patients with anemia as defined had a HR of 2.46 (95%CI 2.08-2.90). ). After adjusting for NCCN-IPI factors, the HR remained significant (1.51; 95%CI 1.29-1.83).
Conclusion
In a population-based cohort of de novo DLBCL patients, we found that pre-therapeutic anemia was present in 49% of the patients and had independent prognostic impact also when adjusted for NCCN-IPI risk factors, and stratified for rituximab treatment. The adverse prognostic value of pretherapeutic anemia was independent of gender and age strata. Our patient cohort was older than the ones of the original NCCN-IPI publications (median age 65 yrs vs. 54 yrs), which probably explains the difference in the proportion of high-risk patients (18% in our cohort vs 8% and 14% in the two original NCCN-IPI cohorts).

Session topic: E-poster
Keyword(s): Anemia, Diffuse large B cell lymphoma, Prognostic factor
Type: Eposter Presentation
Background
Prognosis and treatment options for patients with diffuse large B-cell lymphoma (DLBCL) has for the last 20 years been conveyed on the basis of The International Prognostic Index (IPI). Improving IPI by adding biomarkers is, however, an ongoing discipline. The National Comprehensive Cancer Network IPI (NCCN-IPI) adds to the classical IPI factors more refined age and lactate dehydrogenase (LDH) strata and weighs extranodal disease according to the involvement or not of high risk organs such as bone marrow, CNS, liver/GI tract or lung. The NCCN-IPI was derived and validated on two cohorts with a mean age of 57- and 62-years, respectively, i.e. slightly younger than one would expect in a population-based DLBCL cohort. In Denmark, the universal healthcare system is tax funded and free of charge for all inhabitants ensuring a broad coverage of high quality healthcare. For lymphoma, a national group elaborate management guidelines and a prospective collection of patient-related data stored in the group’s database (LYFO).
Aims
i) To validate the NCCN-IPI in a population-based cohort of de-novo DLBCL patients treated with anthracycline-based chemotherapy; (ii) to analyze, in a multivariate model, whether adding information on pre-therapeutic hemoglobin level improves on the ability of the NCCN-IPI to predict outcome.
Methods
All patients were diagnosed with de novo DLBCL between 2000 and 2012 and treated with anthracycline-based chemotherapy with or without rituximab (R). Patients with indolent- or composite lymphoma were excluded, as were patients with primary CNS-lymphoma. Clinical data were obtained from LYFO and biochemical data were retrieved partly from LYFO and from hospital laboratories. Data on vital status were obtained from the Danish Civil Registration System. Patients were followed from the date of biopsy until death or the end of study (December 10, 2015). Overall survival was described using Kaplan-Meier curves according to NCCN-IPI, treatment and hemoglobin values. Univariate and multivariate analysis were performed using Cox proportional hazard model including NCCN-IPI group, anemia and treatment as covariates with mutual adjustment. Anemia was defined as hemoglobin<7.3 mmol/l l for women and <8.3 mmol/l for men. The proportional hazard assumption was evaluated graphically using log-log plots. The study was approved by the Danish Data Protection Agency (no. 1-16-02-562-13).
Results
A total of 3654 patients fulfilled the inclusion criteria and had a median follow-up of 1888 days. Median age was 65 years (range 15-98), 2033 (56%) were men, 2081 (57%) had Ann Arbor stage III-IV, and 712 (19%) had a performance score ≥2. The pre-therapeutic LDH ratio (measured vs. max reference value) was >1 in 2225 (61%). Anemia was present in 1784 (49%) patients. The NCCN-IPI separated our cohort in low (8%), low intermediate (35%), high intermediate (39%), and high risk (18%) patients.For R-chemo treated patients, the four NCCN-IPI risk groups (low, low-intermediate, high-intermediate, high) revealed 5-year OS values of 0.96 (CI95%, 0.92-0.98), 0.81 (CI95% 0.77-0.83), 0.60 (CI95% 0.56-0.63), and 0.30 (CI95% 0.26-0.37), respectively (Figure 1A). When hemoglobin level was added to the model it showed a significant impact on OS for both intermediate risk groups. Estimated 5 year overall survival in anemic vs. non anemic patients with high-intermediate risk NCCN-IPI was 0.53 (95%CI 0.48-0.58) and 0.69 (95%CI 0.63-0.73) (Figure 1 B) and for low-intermediate 0.74 (95%CI 0.68-0.79) and 0.84 (95%CI 0.80-0.87). Patients with anemia as defined had a HR of 2.46 (95%CI 2.08-2.90). ). After adjusting for NCCN-IPI factors, the HR remained significant (1.51; 95%CI 1.29-1.83).
Conclusion
In a population-based cohort of de novo DLBCL patients, we found that pre-therapeutic anemia was present in 49% of the patients and had independent prognostic impact also when adjusted for NCCN-IPI risk factors, and stratified for rituximab treatment. The adverse prognostic value of pretherapeutic anemia was independent of gender and age strata. Our patient cohort was older than the ones of the original NCCN-IPI publications (median age 65 yrs vs. 54 yrs), which probably explains the difference in the proportion of high-risk patients (18% in our cohort vs 8% and 14% in the two original NCCN-IPI cohorts).

Session topic: E-poster
Keyword(s): Anemia, Diffuse large B cell lymphoma, Prognostic factor
{{ help_message }}
{{filter}}


