EVALUATION OF A PROGNOSTIC MODEL FOR CNS RELAPSE WITHIN THE UK NCRI R-CHOP-14 VS 21 TRIAL
(Abstract release date: 05/19/16)
EHA Library. Mary G. 06/09/16; 132496; E947

Dr. Gleeson Mary
Contributions
Contributions
Abstract
Abstract: E947
Type: Eposter Presentation
Background
Central Nervous System (CNS) relapse of Diffuse Large B-cell Lymphoma (DLBCL) is associated with a poor prognosis. CNS prophylaxis is administered to patients deemed to be at high risk of CNS relapse but the indications for prophylaxis are not standardized. The German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) recently proposed a new 6-factor prognostic model incorporating the 5 International Prognostic Index (IPI) factors in addition to kidney/adrenal gland involvement to determine the risk of CNS relapse in patients with aggressive B-cell lymphoma. This model stratified patients into 3 risk groups: low [0-1 factors, 2 yr CNS relapse risk=0.6% (95% CI 0.0-1.2)]; intermediate [2-3 factors, 2 yr CNS relapse risk=3.4% (95% CI 2.2-4.6)] and high risk [4-6 factors, 2 yr CNS relapse risk=10.2% (95% CI 6.3-14.1)] (Schmitz et al, Lugano 2013); which was subsequently validated in an independent cohort of R-CHOP-treated patients with DLBCL at the British Columbia Cancer Agency (BCCA) (Savage et al, ASH 2014).
Aims
In this analysis we applied the DSHNHL prognostic model to the UK NCRI prospective R-CHOP-14 v 21 trial cohort to determine if similar risk groups could be identified.
Methods
The randomised phase III UK R-CHOP-14 vs 21 trial assessed rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone (R-CHOP) given 2 weekly versus 3 weekly in 1080 DLBCL patients accrued between 2005-2008 (Cunningham et al, 2013). Administration of CNS prophylaxis was at the discretion of investigators but recommended for patients with involvement of bone marrow, peripheral blood, nasal/paranasal sinuses, orbit and testis (12.5mg intrathecal methotrexate (IT MTX) for the first 3 cycles of treatment or according to local guidelines). Details of CNS prophylaxis were retrospectively collected from participating sites using case report forms.
Results
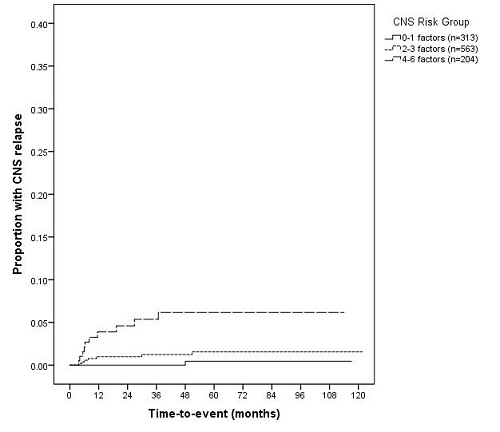
With a median follow-up of 6.5 years the incidence of CNS relapse in our cohort was 1.7% (18/1,080). 17.3% (170/982) of patients in the R-CHOP-14 vs 21 trial received CNS prophylaxis: IT MTX=94.0%, high-dose intravenous MTX=1.3%, prophylaxis type unknown=4.5%. We have previously reported that the incidence of CNS relapse for patients receiving prophylaxis was 3.5% which may suggest some benefit of this therapy (Gleeson et al, ASH 2014). The 6-factor DSHNHL model stratified patients into 3 risk groups for CNS relapse at 2 years: low (0-1 factors) = 0%; intermediate (2-3 factors) = 1.0% (95% CI 0.2-1.8) and high risk (4-6 factors) = 4.6% (95% CI 1.5-7.7) [Figure 1]. Patients developing CNS relapse (n=18) were divided into the following DSHNHL risk groups: low n=1/313, intermediate n=7/563 and high-risk n=10/204. The proportion of patients receiving CNS prophylaxis was 15.3%, 14.2% and 31.4% for low, intermediate and high-risk groups respectively.
Conclusion
The overall incidence of CNS relapse within the R-CHOP-14 vs 21 trial cohort was low (1.7%). The DSHNHL model identified 3 risk groups for CNS relapse at 2 years in the R-CHOP-14 vs 21 cohort, with patients in the high-risk group demonstrating a 2-year incidence of CNS relapse of 4.6%. We observed a lower rate of CNS relapse than that reported by both the DSHNHL and BCCA groups. This may have been influenced by population differences, as the DSHNHL cohort included patients with aggressive B-cell non-Hodgkin lymphoma and the BCCA cohort is a population-based dataset. In addition the proportion of patients receiving CNS prophylaxis may have differed between patient cohorts.
Session topic: E-poster
Keyword(s): CNS, Diffuse large B cell lymphoma, Rituximab
Type: Eposter Presentation
Background
Central Nervous System (CNS) relapse of Diffuse Large B-cell Lymphoma (DLBCL) is associated with a poor prognosis. CNS prophylaxis is administered to patients deemed to be at high risk of CNS relapse but the indications for prophylaxis are not standardized. The German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) recently proposed a new 6-factor prognostic model incorporating the 5 International Prognostic Index (IPI) factors in addition to kidney/adrenal gland involvement to determine the risk of CNS relapse in patients with aggressive B-cell lymphoma. This model stratified patients into 3 risk groups: low [0-1 factors, 2 yr CNS relapse risk=0.6% (95% CI 0.0-1.2)]; intermediate [2-3 factors, 2 yr CNS relapse risk=3.4% (95% CI 2.2-4.6)] and high risk [4-6 factors, 2 yr CNS relapse risk=10.2% (95% CI 6.3-14.1)] (Schmitz et al, Lugano 2013); which was subsequently validated in an independent cohort of R-CHOP-treated patients with DLBCL at the British Columbia Cancer Agency (BCCA) (Savage et al, ASH 2014).
Aims
In this analysis we applied the DSHNHL prognostic model to the UK NCRI prospective R-CHOP-14 v 21 trial cohort to determine if similar risk groups could be identified.
Methods
The randomised phase III UK R-CHOP-14 vs 21 trial assessed rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone (R-CHOP) given 2 weekly versus 3 weekly in 1080 DLBCL patients accrued between 2005-2008 (Cunningham et al, 2013). Administration of CNS prophylaxis was at the discretion of investigators but recommended for patients with involvement of bone marrow, peripheral blood, nasal/paranasal sinuses, orbit and testis (12.5mg intrathecal methotrexate (IT MTX) for the first 3 cycles of treatment or according to local guidelines). Details of CNS prophylaxis were retrospectively collected from participating sites using case report forms.
Results
With a median follow-up of 6.5 years the incidence of CNS relapse in our cohort was 1.7% (18/1,080). 17.3% (170/982) of patients in the R-CHOP-14 vs 21 trial received CNS prophylaxis: IT MTX=94.0%, high-dose intravenous MTX=1.3%, prophylaxis type unknown=4.5%. We have previously reported that the incidence of CNS relapse for patients receiving prophylaxis was 3.5% which may suggest some benefit of this therapy (Gleeson et al, ASH 2014). The 6-factor DSHNHL model stratified patients into 3 risk groups for CNS relapse at 2 years: low (0-1 factors) = 0%; intermediate (2-3 factors) = 1.0% (95% CI 0.2-1.8) and high risk (4-6 factors) = 4.6% (95% CI 1.5-7.7) [Figure 1]. Patients developing CNS relapse (n=18) were divided into the following DSHNHL risk groups: low n=1/313, intermediate n=7/563 and high-risk n=10/204. The proportion of patients receiving CNS prophylaxis was 15.3%, 14.2% and 31.4% for low, intermediate and high-risk groups respectively.
Conclusion
The overall incidence of CNS relapse within the R-CHOP-14 vs 21 trial cohort was low (1.7%). The DSHNHL model identified 3 risk groups for CNS relapse at 2 years in the R-CHOP-14 vs 21 cohort, with patients in the high-risk group demonstrating a 2-year incidence of CNS relapse of 4.6%. We observed a lower rate of CNS relapse than that reported by both the DSHNHL and BCCA groups. This may have been influenced by population differences, as the DSHNHL cohort included patients with aggressive B-cell non-Hodgkin lymphoma and the BCCA cohort is a population-based dataset. In addition the proportion of patients receiving CNS prophylaxis may have differed between patient cohorts.

Session topic: E-poster
Keyword(s): CNS, Diffuse large B cell lymphoma, Rituximab
Abstract: E947
Type: Eposter Presentation
Background
Central Nervous System (CNS) relapse of Diffuse Large B-cell Lymphoma (DLBCL) is associated with a poor prognosis. CNS prophylaxis is administered to patients deemed to be at high risk of CNS relapse but the indications for prophylaxis are not standardized. The German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) recently proposed a new 6-factor prognostic model incorporating the 5 International Prognostic Index (IPI) factors in addition to kidney/adrenal gland involvement to determine the risk of CNS relapse in patients with aggressive B-cell lymphoma. This model stratified patients into 3 risk groups: low [0-1 factors, 2 yr CNS relapse risk=0.6% (95% CI 0.0-1.2)]; intermediate [2-3 factors, 2 yr CNS relapse risk=3.4% (95% CI 2.2-4.6)] and high risk [4-6 factors, 2 yr CNS relapse risk=10.2% (95% CI 6.3-14.1)] (Schmitz et al, Lugano 2013); which was subsequently validated in an independent cohort of R-CHOP-treated patients with DLBCL at the British Columbia Cancer Agency (BCCA) (Savage et al, ASH 2014).
Aims
In this analysis we applied the DSHNHL prognostic model to the UK NCRI prospective R-CHOP-14 v 21 trial cohort to determine if similar risk groups could be identified.
Methods
The randomised phase III UK R-CHOP-14 vs 21 trial assessed rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone (R-CHOP) given 2 weekly versus 3 weekly in 1080 DLBCL patients accrued between 2005-2008 (Cunningham et al, 2013). Administration of CNS prophylaxis was at the discretion of investigators but recommended for patients with involvement of bone marrow, peripheral blood, nasal/paranasal sinuses, orbit and testis (12.5mg intrathecal methotrexate (IT MTX) for the first 3 cycles of treatment or according to local guidelines). Details of CNS prophylaxis were retrospectively collected from participating sites using case report forms.
Results
With a median follow-up of 6.5 years the incidence of CNS relapse in our cohort was 1.7% (18/1,080). 17.3% (170/982) of patients in the R-CHOP-14 vs 21 trial received CNS prophylaxis: IT MTX=94.0%, high-dose intravenous MTX=1.3%, prophylaxis type unknown=4.5%. We have previously reported that the incidence of CNS relapse for patients receiving prophylaxis was 3.5% which may suggest some benefit of this therapy (Gleeson et al, ASH 2014). The 6-factor DSHNHL model stratified patients into 3 risk groups for CNS relapse at 2 years: low (0-1 factors) = 0%; intermediate (2-3 factors) = 1.0% (95% CI 0.2-1.8) and high risk (4-6 factors) = 4.6% (95% CI 1.5-7.7) [Figure 1]. Patients developing CNS relapse (n=18) were divided into the following DSHNHL risk groups: low n=1/313, intermediate n=7/563 and high-risk n=10/204. The proportion of patients receiving CNS prophylaxis was 15.3%, 14.2% and 31.4% for low, intermediate and high-risk groups respectively.
Conclusion
The overall incidence of CNS relapse within the R-CHOP-14 vs 21 trial cohort was low (1.7%). The DSHNHL model identified 3 risk groups for CNS relapse at 2 years in the R-CHOP-14 vs 21 cohort, with patients in the high-risk group demonstrating a 2-year incidence of CNS relapse of 4.6%. We observed a lower rate of CNS relapse than that reported by both the DSHNHL and BCCA groups. This may have been influenced by population differences, as the DSHNHL cohort included patients with aggressive B-cell non-Hodgkin lymphoma and the BCCA cohort is a population-based dataset. In addition the proportion of patients receiving CNS prophylaxis may have differed between patient cohorts.

Session topic: E-poster
Keyword(s): CNS, Diffuse large B cell lymphoma, Rituximab
Type: Eposter Presentation
Background
Central Nervous System (CNS) relapse of Diffuse Large B-cell Lymphoma (DLBCL) is associated with a poor prognosis. CNS prophylaxis is administered to patients deemed to be at high risk of CNS relapse but the indications for prophylaxis are not standardized. The German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) recently proposed a new 6-factor prognostic model incorporating the 5 International Prognostic Index (IPI) factors in addition to kidney/adrenal gland involvement to determine the risk of CNS relapse in patients with aggressive B-cell lymphoma. This model stratified patients into 3 risk groups: low [0-1 factors, 2 yr CNS relapse risk=0.6% (95% CI 0.0-1.2)]; intermediate [2-3 factors, 2 yr CNS relapse risk=3.4% (95% CI 2.2-4.6)] and high risk [4-6 factors, 2 yr CNS relapse risk=10.2% (95% CI 6.3-14.1)] (Schmitz et al, Lugano 2013); which was subsequently validated in an independent cohort of R-CHOP-treated patients with DLBCL at the British Columbia Cancer Agency (BCCA) (Savage et al, ASH 2014).
Aims
In this analysis we applied the DSHNHL prognostic model to the UK NCRI prospective R-CHOP-14 v 21 trial cohort to determine if similar risk groups could be identified.
Methods
The randomised phase III UK R-CHOP-14 vs 21 trial assessed rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone (R-CHOP) given 2 weekly versus 3 weekly in 1080 DLBCL patients accrued between 2005-2008 (Cunningham et al, 2013). Administration of CNS prophylaxis was at the discretion of investigators but recommended for patients with involvement of bone marrow, peripheral blood, nasal/paranasal sinuses, orbit and testis (12.5mg intrathecal methotrexate (IT MTX) for the first 3 cycles of treatment or according to local guidelines). Details of CNS prophylaxis were retrospectively collected from participating sites using case report forms.
Results
With a median follow-up of 6.5 years the incidence of CNS relapse in our cohort was 1.7% (18/1,080). 17.3% (170/982) of patients in the R-CHOP-14 vs 21 trial received CNS prophylaxis: IT MTX=94.0%, high-dose intravenous MTX=1.3%, prophylaxis type unknown=4.5%. We have previously reported that the incidence of CNS relapse for patients receiving prophylaxis was 3.5% which may suggest some benefit of this therapy (Gleeson et al, ASH 2014). The 6-factor DSHNHL model stratified patients into 3 risk groups for CNS relapse at 2 years: low (0-1 factors) = 0%; intermediate (2-3 factors) = 1.0% (95% CI 0.2-1.8) and high risk (4-6 factors) = 4.6% (95% CI 1.5-7.7) [Figure 1]. Patients developing CNS relapse (n=18) were divided into the following DSHNHL risk groups: low n=1/313, intermediate n=7/563 and high-risk n=10/204. The proportion of patients receiving CNS prophylaxis was 15.3%, 14.2% and 31.4% for low, intermediate and high-risk groups respectively.
Conclusion
The overall incidence of CNS relapse within the R-CHOP-14 vs 21 trial cohort was low (1.7%). The DSHNHL model identified 3 risk groups for CNS relapse at 2 years in the R-CHOP-14 vs 21 cohort, with patients in the high-risk group demonstrating a 2-year incidence of CNS relapse of 4.6%. We observed a lower rate of CNS relapse than that reported by both the DSHNHL and BCCA groups. This may have been influenced by population differences, as the DSHNHL cohort included patients with aggressive B-cell non-Hodgkin lymphoma and the BCCA cohort is a population-based dataset. In addition the proportion of patients receiving CNS prophylaxis may have differed between patient cohorts.

Session topic: E-poster
Keyword(s): CNS, Diffuse large B cell lymphoma, Rituximab
{{ help_message }}
{{filter}}


