AGE FACTOR ON REMISSION INDUCTION THERAPY FOR ADULT ACUTE MYELOID LEUKEMIA
(Abstract release date: 05/19/16)
EHA Library. Kim H. 06/09/16; 132486; E937

Prof. Dr. Hawk Kim
Contributions
Contributions
Abstract
Abstract: E937
Type: Eposter Presentation
Background
Therapeutic goal for acute myeloid leukemia (AML) is the cure and usually it can be achieved by intensive chemotherapy. One of the most important factors to choose remission induction therapy is the patient’s age.
Aims
We evaluated the impact of age on remission induction therapy for AML.
Methods
The Korean Society of Hematology AML/MDS Working Party built the adult AML registry. The registry collected 14 year or older patients; however this analysis included only 18 years or older patients as adult AML.
Results
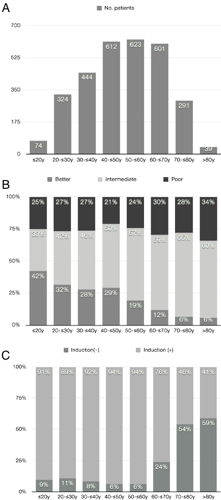
Total 3041 cases were collected in the registry and 3008 adult AML patients were included in this analysis. Enrolled number of patients were presented in Figure 1A. Risk groups based on karyotype showed strikingly different tendency among age groups. Poor risk group had a tendency of slight increase as patients were getting older (21-34%). However more dramatic change was decreasing better risk group instead of increasing intermediate risk group as ages increased (Figure 1B). Performing induction chemotherapy was similar below 60 years ago (89-94%), however it droped to 76% in age 60-70y, 46% in age 70-80y and only 41% in age older than 80y (Figure 1C).We selected 1935 patients who were older than 17y and not acute promyelocytic leukemia and received induction chemotherapy and known induction responses. The complete remission (CR) rates were decreasing as ages increased: 89.5% in age under 20y; 79% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 63% in 60-70y; 58% in 70-80y; 29% in 80y or older patients.When the analysis was focused on patients who had received cytababine-idarubicin (AI) or cytarabine-daunorubicin (AD) induction chemotherapy (n=1377), the CR rates were following: 86% in age under 20y; 77% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 68% in 60-70y; 62% in 70-80y; 50% in 80y or older patients. When analyzing the population according to risk group, the CR rates were similar in better risk group; 92.9% in age under 20y; 100% in 20-30y; 88.6% in 30-40y; 89.2% in 40-50y; 81.6% in 50-60y; 72.2% in 60-70y; 100% in 70-80y patients. In intermediate risk group, the CR rates were 84.6% in age under 20y; 78.9% in 20-30y; 74.1% in 30-40y; 79.4% in 40-50y; 77.6% in 50-60y; 74% in 60-70y; 59.5% in 70-80y; 66.7% in 80y or older patients. CR rates were dramatically dropped in elderly age with poor risk karyotypes; 77.8% in age under 20y; 66.7% in 20-30y; 70.9% in 30-40y; 59.7% in 40-50y; 61.6% in 50-60y; 54.5% in 60-70y; 44.4% in 70-80y; 0% in 80y or older patients. There was no difference of CR rate between AI and AD (p=0.542): p=0.685 in 30-40y; p=0.713 in 40-50y; p=0.452 in 50-60y; p=0.813 in 60-70y; p=1.000 in 70-80y; p=1.000 in age older than 80y. However there was significant higher CR rate among AI regimen in under 30y (p=0.033).
Conclusion
Age clearly impacts on outcome of induction chemotherapy. CR rates were similar in favorable risk group in all age groups. However CR rates of unfavorable risk group dramatically droped in elderly patients. There was no clear difference between AI and AD induction in terms of CR rates. Poor outcome in elderly AML can be resulted mainly from poor risk characteristics.
Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Adult, Age, Induction chemotherapy
Type: Eposter Presentation
Background
Therapeutic goal for acute myeloid leukemia (AML) is the cure and usually it can be achieved by intensive chemotherapy. One of the most important factors to choose remission induction therapy is the patient’s age.
Aims
We evaluated the impact of age on remission induction therapy for AML.
Methods
The Korean Society of Hematology AML/MDS Working Party built the adult AML registry. The registry collected 14 year or older patients; however this analysis included only 18 years or older patients as adult AML.
Results
Total 3041 cases were collected in the registry and 3008 adult AML patients were included in this analysis. Enrolled number of patients were presented in Figure 1A. Risk groups based on karyotype showed strikingly different tendency among age groups. Poor risk group had a tendency of slight increase as patients were getting older (21-34%). However more dramatic change was decreasing better risk group instead of increasing intermediate risk group as ages increased (Figure 1B). Performing induction chemotherapy was similar below 60 years ago (89-94%), however it droped to 76% in age 60-70y, 46% in age 70-80y and only 41% in age older than 80y (Figure 1C).We selected 1935 patients who were older than 17y and not acute promyelocytic leukemia and received induction chemotherapy and known induction responses. The complete remission (CR) rates were decreasing as ages increased: 89.5% in age under 20y; 79% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 63% in 60-70y; 58% in 70-80y; 29% in 80y or older patients.When the analysis was focused on patients who had received cytababine-idarubicin (AI) or cytarabine-daunorubicin (AD) induction chemotherapy (n=1377), the CR rates were following: 86% in age under 20y; 77% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 68% in 60-70y; 62% in 70-80y; 50% in 80y or older patients. When analyzing the population according to risk group, the CR rates were similar in better risk group; 92.9% in age under 20y; 100% in 20-30y; 88.6% in 30-40y; 89.2% in 40-50y; 81.6% in 50-60y; 72.2% in 60-70y; 100% in 70-80y patients. In intermediate risk group, the CR rates were 84.6% in age under 20y; 78.9% in 20-30y; 74.1% in 30-40y; 79.4% in 40-50y; 77.6% in 50-60y; 74% in 60-70y; 59.5% in 70-80y; 66.7% in 80y or older patients. CR rates were dramatically dropped in elderly age with poor risk karyotypes; 77.8% in age under 20y; 66.7% in 20-30y; 70.9% in 30-40y; 59.7% in 40-50y; 61.6% in 50-60y; 54.5% in 60-70y; 44.4% in 70-80y; 0% in 80y or older patients. There was no difference of CR rate between AI and AD (p=0.542): p=0.685 in 30-40y; p=0.713 in 40-50y; p=0.452 in 50-60y; p=0.813 in 60-70y; p=1.000 in 70-80y; p=1.000 in age older than 80y. However there was significant higher CR rate among AI regimen in under 30y (p=0.033).
Conclusion
Age clearly impacts on outcome of induction chemotherapy. CR rates were similar in favorable risk group in all age groups. However CR rates of unfavorable risk group dramatically droped in elderly patients. There was no clear difference between AI and AD induction in terms of CR rates. Poor outcome in elderly AML can be resulted mainly from poor risk characteristics.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Adult, Age, Induction chemotherapy
Abstract: E937
Type: Eposter Presentation
Background
Therapeutic goal for acute myeloid leukemia (AML) is the cure and usually it can be achieved by intensive chemotherapy. One of the most important factors to choose remission induction therapy is the patient’s age.
Aims
We evaluated the impact of age on remission induction therapy for AML.
Methods
The Korean Society of Hematology AML/MDS Working Party built the adult AML registry. The registry collected 14 year or older patients; however this analysis included only 18 years or older patients as adult AML.
Results
Total 3041 cases were collected in the registry and 3008 adult AML patients were included in this analysis. Enrolled number of patients were presented in Figure 1A. Risk groups based on karyotype showed strikingly different tendency among age groups. Poor risk group had a tendency of slight increase as patients were getting older (21-34%). However more dramatic change was decreasing better risk group instead of increasing intermediate risk group as ages increased (Figure 1B). Performing induction chemotherapy was similar below 60 years ago (89-94%), however it droped to 76% in age 60-70y, 46% in age 70-80y and only 41% in age older than 80y (Figure 1C).We selected 1935 patients who were older than 17y and not acute promyelocytic leukemia and received induction chemotherapy and known induction responses. The complete remission (CR) rates were decreasing as ages increased: 89.5% in age under 20y; 79% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 63% in 60-70y; 58% in 70-80y; 29% in 80y or older patients.When the analysis was focused on patients who had received cytababine-idarubicin (AI) or cytarabine-daunorubicin (AD) induction chemotherapy (n=1377), the CR rates were following: 86% in age under 20y; 77% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 68% in 60-70y; 62% in 70-80y; 50% in 80y or older patients. When analyzing the population according to risk group, the CR rates were similar in better risk group; 92.9% in age under 20y; 100% in 20-30y; 88.6% in 30-40y; 89.2% in 40-50y; 81.6% in 50-60y; 72.2% in 60-70y; 100% in 70-80y patients. In intermediate risk group, the CR rates were 84.6% in age under 20y; 78.9% in 20-30y; 74.1% in 30-40y; 79.4% in 40-50y; 77.6% in 50-60y; 74% in 60-70y; 59.5% in 70-80y; 66.7% in 80y or older patients. CR rates were dramatically dropped in elderly age with poor risk karyotypes; 77.8% in age under 20y; 66.7% in 20-30y; 70.9% in 30-40y; 59.7% in 40-50y; 61.6% in 50-60y; 54.5% in 60-70y; 44.4% in 70-80y; 0% in 80y or older patients. There was no difference of CR rate between AI and AD (p=0.542): p=0.685 in 30-40y; p=0.713 in 40-50y; p=0.452 in 50-60y; p=0.813 in 60-70y; p=1.000 in 70-80y; p=1.000 in age older than 80y. However there was significant higher CR rate among AI regimen in under 30y (p=0.033).
Conclusion
Age clearly impacts on outcome of induction chemotherapy. CR rates were similar in favorable risk group in all age groups. However CR rates of unfavorable risk group dramatically droped in elderly patients. There was no clear difference between AI and AD induction in terms of CR rates. Poor outcome in elderly AML can be resulted mainly from poor risk characteristics.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Adult, Age, Induction chemotherapy
Type: Eposter Presentation
Background
Therapeutic goal for acute myeloid leukemia (AML) is the cure and usually it can be achieved by intensive chemotherapy. One of the most important factors to choose remission induction therapy is the patient’s age.
Aims
We evaluated the impact of age on remission induction therapy for AML.
Methods
The Korean Society of Hematology AML/MDS Working Party built the adult AML registry. The registry collected 14 year or older patients; however this analysis included only 18 years or older patients as adult AML.
Results
Total 3041 cases were collected in the registry and 3008 adult AML patients were included in this analysis. Enrolled number of patients were presented in Figure 1A. Risk groups based on karyotype showed strikingly different tendency among age groups. Poor risk group had a tendency of slight increase as patients were getting older (21-34%). However more dramatic change was decreasing better risk group instead of increasing intermediate risk group as ages increased (Figure 1B). Performing induction chemotherapy was similar below 60 years ago (89-94%), however it droped to 76% in age 60-70y, 46% in age 70-80y and only 41% in age older than 80y (Figure 1C).We selected 1935 patients who were older than 17y and not acute promyelocytic leukemia and received induction chemotherapy and known induction responses. The complete remission (CR) rates were decreasing as ages increased: 89.5% in age under 20y; 79% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 63% in 60-70y; 58% in 70-80y; 29% in 80y or older patients.When the analysis was focused on patients who had received cytababine-idarubicin (AI) or cytarabine-daunorubicin (AD) induction chemotherapy (n=1377), the CR rates were following: 86% in age under 20y; 77% in 20-30y; 75% in 30-40y; 76% in 40-50y; 73% in 50-60y; 68% in 60-70y; 62% in 70-80y; 50% in 80y or older patients. When analyzing the population according to risk group, the CR rates were similar in better risk group; 92.9% in age under 20y; 100% in 20-30y; 88.6% in 30-40y; 89.2% in 40-50y; 81.6% in 50-60y; 72.2% in 60-70y; 100% in 70-80y patients. In intermediate risk group, the CR rates were 84.6% in age under 20y; 78.9% in 20-30y; 74.1% in 30-40y; 79.4% in 40-50y; 77.6% in 50-60y; 74% in 60-70y; 59.5% in 70-80y; 66.7% in 80y or older patients. CR rates were dramatically dropped in elderly age with poor risk karyotypes; 77.8% in age under 20y; 66.7% in 20-30y; 70.9% in 30-40y; 59.7% in 40-50y; 61.6% in 50-60y; 54.5% in 60-70y; 44.4% in 70-80y; 0% in 80y or older patients. There was no difference of CR rate between AI and AD (p=0.542): p=0.685 in 30-40y; p=0.713 in 40-50y; p=0.452 in 50-60y; p=0.813 in 60-70y; p=1.000 in 70-80y; p=1.000 in age older than 80y. However there was significant higher CR rate among AI regimen in under 30y (p=0.033).
Conclusion
Age clearly impacts on outcome of induction chemotherapy. CR rates were similar in favorable risk group in all age groups. However CR rates of unfavorable risk group dramatically droped in elderly patients. There was no clear difference between AI and AD induction in terms of CR rates. Poor outcome in elderly AML can be resulted mainly from poor risk characteristics.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Adult, Age, Induction chemotherapy
{{ help_message }}
{{filter}}


