CHARACTERIZATION OF PATIENTS WITH RELAPSED OR REFRACTORY AML IN CONTINUED FOLLOW-UP AFTER TREATMENT WITH VOSAROXIN/CYTARABINE VS PLACEBO/CYTARABINE IN THE VALOR TRIAL
(Abstract release date: 05/19/16)
EHA Library. Pigneux A. 06/09/16; 132479; E930

Ms. Arnaud Pigneux
Contributions
Contributions
Abstract
Abstract: E930
Type: Eposter Presentation
Background
Patients with relapsed/refractory (R/R) AML have a median overall survival (OS) less than 1 year. In the phase 3 VALOR trial, vosaroxin/cytarabine prolonged median OS in patients with R/R AML by 1.4 months vs placebo/cytarabine (7.5 vs 6.1 months; HR = 0.87 [95% CI 0.73-1.02]; P = 0.061). Of 711 enrolled patients, 134 (19%) were alive in follow-up at the primary analysis. After the primary analysis, ongoing patients were followed for survival.
Aims
To characterize patients who continue to be followed for survival in the VALOR trial.
Methods
In VALOR, patients with R/R AML were randomized 1:1 to receive cytarabine (1 g/m2 IV over 2 h, d 1-5) plus either vosaroxin (90 mg/m2 IV over 10 min d 1, 4; 70 mg/m2 in subsequent cycles) or placebo.
Results
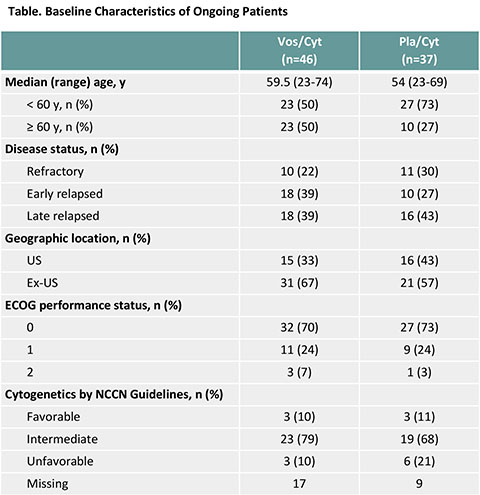
As of Jan 22, 2016, 83 patients (12%) were alive in follow-up: 46/356 (13%) in the vosaroxin/cytarabine arm and 37/355 (10%) in the placebo/cytarabine arm. Median follow-up in these patients was 40 months (range 28-60). Patient characteristics are presented (Table); a higher proportion of patients were ≥ 60 years in the vosaroxin/cytarabine arm (50% vs 27% with placebo/cytarabine). Most achieved complete remission (CR) on study (70% with vosaroxin/cytarabine; 51% with placebo/cytarabine); over half maintained CR at database lock (59% with vosaroxin/cytarabine; 49% with placebo/cytarabine). Nearly all received subsequent therapy (93% with vosaroxin/cytarabine; 100% with placebo/cytarabine). Most patients on vosaroxin/cytarabine (85%) and all patients on placebo/cytarabine received posttreatment stem cell transplantation (SCT). Seven patients in the vosaroxin/cytarabine arm did not undergo SCT; all were ≥ 60 years of age. Median follow-up in these 7 patients was 33 months (range 31-48).
Conclusion
A small proportion of patients with R/R AML continue to be followed for survival in VALOR. Typically, these patients achieved CR followed by SCT; however, some patients ≥ 60 years treated with vosaroxin/cytarabine achieved long-term survival without SCT.
Session topic: E-poster
Type: Eposter Presentation
Background
Patients with relapsed/refractory (R/R) AML have a median overall survival (OS) less than 1 year. In the phase 3 VALOR trial, vosaroxin/cytarabine prolonged median OS in patients with R/R AML by 1.4 months vs placebo/cytarabine (7.5 vs 6.1 months; HR = 0.87 [95% CI 0.73-1.02]; P = 0.061). Of 711 enrolled patients, 134 (19%) were alive in follow-up at the primary analysis. After the primary analysis, ongoing patients were followed for survival.
Aims
To characterize patients who continue to be followed for survival in the VALOR trial.
Methods
In VALOR, patients with R/R AML were randomized 1:1 to receive cytarabine (1 g/m2 IV over 2 h, d 1-5) plus either vosaroxin (90 mg/m2 IV over 10 min d 1, 4; 70 mg/m2 in subsequent cycles) or placebo.
Results
As of Jan 22, 2016, 83 patients (12%) were alive in follow-up: 46/356 (13%) in the vosaroxin/cytarabine arm and 37/355 (10%) in the placebo/cytarabine arm. Median follow-up in these patients was 40 months (range 28-60). Patient characteristics are presented (Table); a higher proportion of patients were ≥ 60 years in the vosaroxin/cytarabine arm (50% vs 27% with placebo/cytarabine). Most achieved complete remission (CR) on study (70% with vosaroxin/cytarabine; 51% with placebo/cytarabine); over half maintained CR at database lock (59% with vosaroxin/cytarabine; 49% with placebo/cytarabine). Nearly all received subsequent therapy (93% with vosaroxin/cytarabine; 100% with placebo/cytarabine). Most patients on vosaroxin/cytarabine (85%) and all patients on placebo/cytarabine received posttreatment stem cell transplantation (SCT). Seven patients in the vosaroxin/cytarabine arm did not undergo SCT; all were ≥ 60 years of age. Median follow-up in these 7 patients was 33 months (range 31-48).
Conclusion
A small proportion of patients with R/R AML continue to be followed for survival in VALOR. Typically, these patients achieved CR followed by SCT; however, some patients ≥ 60 years treated with vosaroxin/cytarabine achieved long-term survival without SCT.

Session topic: E-poster
Abstract: E930
Type: Eposter Presentation
Background
Patients with relapsed/refractory (R/R) AML have a median overall survival (OS) less than 1 year. In the phase 3 VALOR trial, vosaroxin/cytarabine prolonged median OS in patients with R/R AML by 1.4 months vs placebo/cytarabine (7.5 vs 6.1 months; HR = 0.87 [95% CI 0.73-1.02]; P = 0.061). Of 711 enrolled patients, 134 (19%) were alive in follow-up at the primary analysis. After the primary analysis, ongoing patients were followed for survival.
Aims
To characterize patients who continue to be followed for survival in the VALOR trial.
Methods
In VALOR, patients with R/R AML were randomized 1:1 to receive cytarabine (1 g/m2 IV over 2 h, d 1-5) plus either vosaroxin (90 mg/m2 IV over 10 min d 1, 4; 70 mg/m2 in subsequent cycles) or placebo.
Results
As of Jan 22, 2016, 83 patients (12%) were alive in follow-up: 46/356 (13%) in the vosaroxin/cytarabine arm and 37/355 (10%) in the placebo/cytarabine arm. Median follow-up in these patients was 40 months (range 28-60). Patient characteristics are presented (Table); a higher proportion of patients were ≥ 60 years in the vosaroxin/cytarabine arm (50% vs 27% with placebo/cytarabine). Most achieved complete remission (CR) on study (70% with vosaroxin/cytarabine; 51% with placebo/cytarabine); over half maintained CR at database lock (59% with vosaroxin/cytarabine; 49% with placebo/cytarabine). Nearly all received subsequent therapy (93% with vosaroxin/cytarabine; 100% with placebo/cytarabine). Most patients on vosaroxin/cytarabine (85%) and all patients on placebo/cytarabine received posttreatment stem cell transplantation (SCT). Seven patients in the vosaroxin/cytarabine arm did not undergo SCT; all were ≥ 60 years of age. Median follow-up in these 7 patients was 33 months (range 31-48).
Conclusion
A small proportion of patients with R/R AML continue to be followed for survival in VALOR. Typically, these patients achieved CR followed by SCT; however, some patients ≥ 60 years treated with vosaroxin/cytarabine achieved long-term survival without SCT.

Session topic: E-poster
Type: Eposter Presentation
Background
Patients with relapsed/refractory (R/R) AML have a median overall survival (OS) less than 1 year. In the phase 3 VALOR trial, vosaroxin/cytarabine prolonged median OS in patients with R/R AML by 1.4 months vs placebo/cytarabine (7.5 vs 6.1 months; HR = 0.87 [95% CI 0.73-1.02]; P = 0.061). Of 711 enrolled patients, 134 (19%) were alive in follow-up at the primary analysis. After the primary analysis, ongoing patients were followed for survival.
Aims
To characterize patients who continue to be followed for survival in the VALOR trial.
Methods
In VALOR, patients with R/R AML were randomized 1:1 to receive cytarabine (1 g/m2 IV over 2 h, d 1-5) plus either vosaroxin (90 mg/m2 IV over 10 min d 1, 4; 70 mg/m2 in subsequent cycles) or placebo.
Results
As of Jan 22, 2016, 83 patients (12%) were alive in follow-up: 46/356 (13%) in the vosaroxin/cytarabine arm and 37/355 (10%) in the placebo/cytarabine arm. Median follow-up in these patients was 40 months (range 28-60). Patient characteristics are presented (Table); a higher proportion of patients were ≥ 60 years in the vosaroxin/cytarabine arm (50% vs 27% with placebo/cytarabine). Most achieved complete remission (CR) on study (70% with vosaroxin/cytarabine; 51% with placebo/cytarabine); over half maintained CR at database lock (59% with vosaroxin/cytarabine; 49% with placebo/cytarabine). Nearly all received subsequent therapy (93% with vosaroxin/cytarabine; 100% with placebo/cytarabine). Most patients on vosaroxin/cytarabine (85%) and all patients on placebo/cytarabine received posttreatment stem cell transplantation (SCT). Seven patients in the vosaroxin/cytarabine arm did not undergo SCT; all were ≥ 60 years of age. Median follow-up in these 7 patients was 33 months (range 31-48).
Conclusion
A small proportion of patients with R/R AML continue to be followed for survival in VALOR. Typically, these patients achieved CR followed by SCT; however, some patients ≥ 60 years treated with vosaroxin/cytarabine achieved long-term survival without SCT.

Session topic: E-poster
{{ help_message }}
{{filter}}


