CLINICAL CHARACTERISTICS AND OUTCOME OF PEDIATRIC ACUTE MYELOID LEUKEMIA WITH DEL(5Q) : A REPORT FROM THE JAPANESE PEDIATRIC LEUKEMIA/LYMPHOMA STUDY GROUP.
(Abstract release date: 05/19/16)
EHA Library. Kinoshita A. 06/09/16; 132467; E918

Prof. Dr. Akitoshi Kinoshita
Contributions
Contributions
Abstract
Abstract: E918
Type: Eposter Presentation
Background
Deletion of chromosome 5q (5q-) confer a poor prognosis in adults with acute myeloid leukemia (AML) and associated with higher white blood cell count at diagnosis. However, since this chromosomal abnormality is rare in pediatric patients with AML, the clinical characteristics and prognostic significance are not clear.
Aims
To clarify AML with 5q- in terms of hematological and clinical characteristics, and prognosis.
Methods
Between November 2006 and December 2010, 485 consecutive patients aged < 18 years with suspected AML excluding acute promyelocytic leukemia, Down syndrome, secondary AML, myeloid/natural killer cell leukemia, and myeloid sarcoma were registered in AML-05 conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group. The diagnosis according to the 2008 WHO classification was determined centrally by integrating morphologic, cytogenetic, immunologic, molecular and clinical parameters. We stratified patients after the second induction course to either of the three risk groups according to the cytogenetics and FLT3-ITD status at diagnosis and the response to induction chemotherapy. Allogeneic hematopoietic transplantation (HSCT) was planned for the patients allocated for the high risk group including 5q- after the third or fourth chemotherapy course.
Results
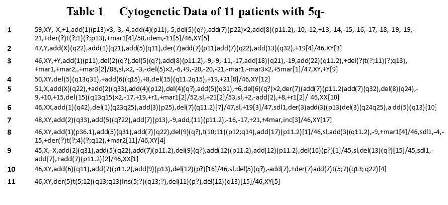
Of the 485 patients registered, 32 patients were excluded because of misdiagnosis or refusal by the guardians. Of the 453 eligible patients, a total of 11 patients (2.4 %) were identified as AML with 5q-. The table 1 shows cytogenetic data for the 5q- patients. All patients with 5q- had unrelated chromosomal abnormalities > 3 and were therefore diagnosed as AML with myelodysplasia-related changes. Median age at diagnosis was 1.9 years (range, 0.2-13.8) and median WBC count at presentation was 28.1×109/l(range, 2.1-50.7). Any significant differences were seen in age and WBC count compared to those without 5q-. Of the 11 patients, 7 (64%) had a complete response (CR) to induction chemotherapy. Of the 4 patients with induction failure, 2 were treated with HSCT and were alive. Of the 7 patients achieving CR, bone marrow relapse was recognized in 5, 2 of whom were alive after HSCT. Excluding patients with core binding factor leukemia, the 3-year event-free survival for patients with 5q- was significantly lower than for those without 5q- (18% versus 56%, P=0.02). However, the 3-year overall survival was not significantly different between patients with 5q- and those without 5q- (54% versus 70%, P=0.07).
Conclusion
Our study suggests that AML with 5q- has a poor prognosis, but that intensive therapy including HSCT may improve outcome in children with AML with 5q-.
Session topic: E-poster
Type: Eposter Presentation
Background
Deletion of chromosome 5q (5q-) confer a poor prognosis in adults with acute myeloid leukemia (AML) and associated with higher white blood cell count at diagnosis. However, since this chromosomal abnormality is rare in pediatric patients with AML, the clinical characteristics and prognostic significance are not clear.
Aims
To clarify AML with 5q- in terms of hematological and clinical characteristics, and prognosis.
Methods
Between November 2006 and December 2010, 485 consecutive patients aged < 18 years with suspected AML excluding acute promyelocytic leukemia, Down syndrome, secondary AML, myeloid/natural killer cell leukemia, and myeloid sarcoma were registered in AML-05 conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group. The diagnosis according to the 2008 WHO classification was determined centrally by integrating morphologic, cytogenetic, immunologic, molecular and clinical parameters. We stratified patients after the second induction course to either of the three risk groups according to the cytogenetics and FLT3-ITD status at diagnosis and the response to induction chemotherapy. Allogeneic hematopoietic transplantation (HSCT) was planned for the patients allocated for the high risk group including 5q- after the third or fourth chemotherapy course.
Results
Of the 485 patients registered, 32 patients were excluded because of misdiagnosis or refusal by the guardians. Of the 453 eligible patients, a total of 11 patients (2.4 %) were identified as AML with 5q-. The table 1 shows cytogenetic data for the 5q- patients. All patients with 5q- had unrelated chromosomal abnormalities > 3 and were therefore diagnosed as AML with myelodysplasia-related changes. Median age at diagnosis was 1.9 years (range, 0.2-13.8) and median WBC count at presentation was 28.1×109/l(range, 2.1-50.7). Any significant differences were seen in age and WBC count compared to those without 5q-. Of the 11 patients, 7 (64%) had a complete response (CR) to induction chemotherapy. Of the 4 patients with induction failure, 2 were treated with HSCT and were alive. Of the 7 patients achieving CR, bone marrow relapse was recognized in 5, 2 of whom were alive after HSCT. Excluding patients with core binding factor leukemia, the 3-year event-free survival for patients with 5q- was significantly lower than for those without 5q- (18% versus 56%, P=0.02). However, the 3-year overall survival was not significantly different between patients with 5q- and those without 5q- (54% versus 70%, P=0.07).
Conclusion
Our study suggests that AML with 5q- has a poor prognosis, but that intensive therapy including HSCT may improve outcome in children with AML with 5q-.

Session topic: E-poster
Abstract: E918
Type: Eposter Presentation
Background
Deletion of chromosome 5q (5q-) confer a poor prognosis in adults with acute myeloid leukemia (AML) and associated with higher white blood cell count at diagnosis. However, since this chromosomal abnormality is rare in pediatric patients with AML, the clinical characteristics and prognostic significance are not clear.
Aims
To clarify AML with 5q- in terms of hematological and clinical characteristics, and prognosis.
Methods
Between November 2006 and December 2010, 485 consecutive patients aged < 18 years with suspected AML excluding acute promyelocytic leukemia, Down syndrome, secondary AML, myeloid/natural killer cell leukemia, and myeloid sarcoma were registered in AML-05 conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group. The diagnosis according to the 2008 WHO classification was determined centrally by integrating morphologic, cytogenetic, immunologic, molecular and clinical parameters. We stratified patients after the second induction course to either of the three risk groups according to the cytogenetics and FLT3-ITD status at diagnosis and the response to induction chemotherapy. Allogeneic hematopoietic transplantation (HSCT) was planned for the patients allocated for the high risk group including 5q- after the third or fourth chemotherapy course.
Results
Of the 485 patients registered, 32 patients were excluded because of misdiagnosis or refusal by the guardians. Of the 453 eligible patients, a total of 11 patients (2.4 %) were identified as AML with 5q-. The table 1 shows cytogenetic data for the 5q- patients. All patients with 5q- had unrelated chromosomal abnormalities > 3 and were therefore diagnosed as AML with myelodysplasia-related changes. Median age at diagnosis was 1.9 years (range, 0.2-13.8) and median WBC count at presentation was 28.1×109/l(range, 2.1-50.7). Any significant differences were seen in age and WBC count compared to those without 5q-. Of the 11 patients, 7 (64%) had a complete response (CR) to induction chemotherapy. Of the 4 patients with induction failure, 2 were treated with HSCT and were alive. Of the 7 patients achieving CR, bone marrow relapse was recognized in 5, 2 of whom were alive after HSCT. Excluding patients with core binding factor leukemia, the 3-year event-free survival for patients with 5q- was significantly lower than for those without 5q- (18% versus 56%, P=0.02). However, the 3-year overall survival was not significantly different between patients with 5q- and those without 5q- (54% versus 70%, P=0.07).
Conclusion
Our study suggests that AML with 5q- has a poor prognosis, but that intensive therapy including HSCT may improve outcome in children with AML with 5q-.

Session topic: E-poster
Type: Eposter Presentation
Background
Deletion of chromosome 5q (5q-) confer a poor prognosis in adults with acute myeloid leukemia (AML) and associated with higher white blood cell count at diagnosis. However, since this chromosomal abnormality is rare in pediatric patients with AML, the clinical characteristics and prognostic significance are not clear.
Aims
To clarify AML with 5q- in terms of hematological and clinical characteristics, and prognosis.
Methods
Between November 2006 and December 2010, 485 consecutive patients aged < 18 years with suspected AML excluding acute promyelocytic leukemia, Down syndrome, secondary AML, myeloid/natural killer cell leukemia, and myeloid sarcoma were registered in AML-05 conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group. The diagnosis according to the 2008 WHO classification was determined centrally by integrating morphologic, cytogenetic, immunologic, molecular and clinical parameters. We stratified patients after the second induction course to either of the three risk groups according to the cytogenetics and FLT3-ITD status at diagnosis and the response to induction chemotherapy. Allogeneic hematopoietic transplantation (HSCT) was planned for the patients allocated for the high risk group including 5q- after the third or fourth chemotherapy course.
Results
Of the 485 patients registered, 32 patients were excluded because of misdiagnosis or refusal by the guardians. Of the 453 eligible patients, a total of 11 patients (2.4 %) were identified as AML with 5q-. The table 1 shows cytogenetic data for the 5q- patients. All patients with 5q- had unrelated chromosomal abnormalities > 3 and were therefore diagnosed as AML with myelodysplasia-related changes. Median age at diagnosis was 1.9 years (range, 0.2-13.8) and median WBC count at presentation was 28.1×109/l(range, 2.1-50.7). Any significant differences were seen in age and WBC count compared to those without 5q-. Of the 11 patients, 7 (64%) had a complete response (CR) to induction chemotherapy. Of the 4 patients with induction failure, 2 were treated with HSCT and were alive. Of the 7 patients achieving CR, bone marrow relapse was recognized in 5, 2 of whom were alive after HSCT. Excluding patients with core binding factor leukemia, the 3-year event-free survival for patients with 5q- was significantly lower than for those without 5q- (18% versus 56%, P=0.02). However, the 3-year overall survival was not significantly different between patients with 5q- and those without 5q- (54% versus 70%, P=0.07).
Conclusion
Our study suggests that AML with 5q- has a poor prognosis, but that intensive therapy including HSCT may improve outcome in children with AML with 5q-.

Session topic: E-poster
{{ help_message }}
{{filter}}


