REPOSITIONING OF QUINACRINE FOR TREATMENT OF ACUTE MYELOID LEUKEMIA - SYNERGIES AND IN VIVO EFFECTS
(Abstract release date: 05/19/16)
EHA Library. Eriksson A. 06/09/16; 132447; E898
Disclosure(s): RL, PN and MF are co-founders and shareholders of Repos Pharma AB, a small Swedish research and development company dedicated to investigations of drug repositioning in the cancer area.

Dr. Anna Eriksson
Contributions
Contributions
Abstract
Abstract: E898
Type: Eposter Presentation
Background
We have previously reported that quinacrine, formerly extensively used as an antimalarial drug, may have repositioning potential for treatment of acute myeloid leukemia (AML)1.
Aims
The aim of this study was to further evaluate the potential of quinacrine for a clinical trial in AML by investigating its possible synergistic effect with other antileukemic compounds as well as evaluating its efficacy in vivo in a mouse model.
Methods
Cytotoxic activity of quinacrine in combination with one of 9 different drugs (daunorubicin, cytarabine, azacitidine, decitabine, sorafenib, geldanamycin, All-Trans Retinoic Acid (ATRA), vorinostat, and arsenic trioxide) in AML cell lines was evaluated using the fluorometric microculture cytotoxicity assay (FMCA). Assessment of combinatorial effects and possible synergy was performed using conventional Bliss independence analysis based on results from all combinations of 4 different concentrations (including zero concentration) of each drug.Activity of quinacrine in vivo was investigated in a model with AML-PS cells injected intravenously in female SCID mice (at Accelera S.r.l). After two days, groups of ten mice were randomized to treatment with quinacrine or vehicle control. Quinacrine was administered by oral gavage at the dose of 100 mg/kg three times a week for two consecutive weeks; control animals were treated with vehicle (iv) only (twice a week for two weeks). Mice were monitored daily for mortality and clinical signs, body weights were evaluated twice a week. Blood aliquots were collected on days 30 and 31 to determine the percentage of circulating leukemic cells by FACS analysis. BD Cellquest software was used for data collection and analysis. Median survival time (MST) was calculated and the log-rank test was used to evaluate the statistical significance of differences between the control group vs. the quinacrine-treated group.
Results
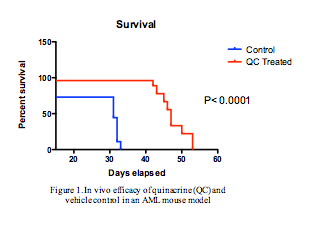
In the in vitro drug combination analysis, several promising synergies were observed after studying duplicated experiments, for instance when combining quinacrine with geldanamycin, ATRA, cytarabine, hypomethylating agents or sorafenib.In the AML mouse model, evaluation of circulating leukemic cells detected in blood samples (in percent of white blood cells) showed 72 % human tumor cells in the control mice, whereas in mice treated with quinacrine, this was only 2.2%. In agreement with these data, the MST of control mice was 34 days whereas it was 46 days in quinacrine-treated mice (p < 0.0001). At the tested dose, quinacrine did not decrease the body weight of treated mice.
Conclusion
These results strengthen the repositioning potential for quinacrine in AML, suggesting in vivo efficacy as well as promising synergies in combination with different agents, including geldanamycin, conventional chemotherapeutics as well as the hypomethylating agent azacitidine. These results provide further support for evaluation of the anti-leukemic effect of quinacrine in a clinical trial in AML. References1.Eriksson A, Osterroos A, Hassan S, et al. Drug screen in patient cells suggests quinacrine to be repositioned for treatment of acute myeloid leukemia. Blood cancer journal 2015; 5: e307.
Session topic: E-poster
Keyword(s): AML
Type: Eposter Presentation
Background
We have previously reported that quinacrine, formerly extensively used as an antimalarial drug, may have repositioning potential for treatment of acute myeloid leukemia (AML)1.
Aims
The aim of this study was to further evaluate the potential of quinacrine for a clinical trial in AML by investigating its possible synergistic effect with other antileukemic compounds as well as evaluating its efficacy in vivo in a mouse model.
Methods
Cytotoxic activity of quinacrine in combination with one of 9 different drugs (daunorubicin, cytarabine, azacitidine, decitabine, sorafenib, geldanamycin, All-Trans Retinoic Acid (ATRA), vorinostat, and arsenic trioxide) in AML cell lines was evaluated using the fluorometric microculture cytotoxicity assay (FMCA). Assessment of combinatorial effects and possible synergy was performed using conventional Bliss independence analysis based on results from all combinations of 4 different concentrations (including zero concentration) of each drug.Activity of quinacrine in vivo was investigated in a model with AML-PS cells injected intravenously in female SCID mice (at Accelera S.r.l). After two days, groups of ten mice were randomized to treatment with quinacrine or vehicle control. Quinacrine was administered by oral gavage at the dose of 100 mg/kg three times a week for two consecutive weeks; control animals were treated with vehicle (iv) only (twice a week for two weeks). Mice were monitored daily for mortality and clinical signs, body weights were evaluated twice a week. Blood aliquots were collected on days 30 and 31 to determine the percentage of circulating leukemic cells by FACS analysis. BD Cellquest software was used for data collection and analysis. Median survival time (MST) was calculated and the log-rank test was used to evaluate the statistical significance of differences between the control group vs. the quinacrine-treated group.
Results
In the in vitro drug combination analysis, several promising synergies were observed after studying duplicated experiments, for instance when combining quinacrine with geldanamycin, ATRA, cytarabine, hypomethylating agents or sorafenib.In the AML mouse model, evaluation of circulating leukemic cells detected in blood samples (in percent of white blood cells) showed 72 % human tumor cells in the control mice, whereas in mice treated with quinacrine, this was only 2.2%. In agreement with these data, the MST of control mice was 34 days whereas it was 46 days in quinacrine-treated mice (p < 0.0001). At the tested dose, quinacrine did not decrease the body weight of treated mice.
Conclusion
These results strengthen the repositioning potential for quinacrine in AML, suggesting in vivo efficacy as well as promising synergies in combination with different agents, including geldanamycin, conventional chemotherapeutics as well as the hypomethylating agent azacitidine. These results provide further support for evaluation of the anti-leukemic effect of quinacrine in a clinical trial in AML. References1.Eriksson A, Osterroos A, Hassan S, et al. Drug screen in patient cells suggests quinacrine to be repositioned for treatment of acute myeloid leukemia. Blood cancer journal 2015; 5: e307.

Session topic: E-poster
Keyword(s): AML
Abstract: E898
Type: Eposter Presentation
Background
We have previously reported that quinacrine, formerly extensively used as an antimalarial drug, may have repositioning potential for treatment of acute myeloid leukemia (AML)1.
Aims
The aim of this study was to further evaluate the potential of quinacrine for a clinical trial in AML by investigating its possible synergistic effect with other antileukemic compounds as well as evaluating its efficacy in vivo in a mouse model.
Methods
Cytotoxic activity of quinacrine in combination with one of 9 different drugs (daunorubicin, cytarabine, azacitidine, decitabine, sorafenib, geldanamycin, All-Trans Retinoic Acid (ATRA), vorinostat, and arsenic trioxide) in AML cell lines was evaluated using the fluorometric microculture cytotoxicity assay (FMCA). Assessment of combinatorial effects and possible synergy was performed using conventional Bliss independence analysis based on results from all combinations of 4 different concentrations (including zero concentration) of each drug.Activity of quinacrine in vivo was investigated in a model with AML-PS cells injected intravenously in female SCID mice (at Accelera S.r.l). After two days, groups of ten mice were randomized to treatment with quinacrine or vehicle control. Quinacrine was administered by oral gavage at the dose of 100 mg/kg three times a week for two consecutive weeks; control animals were treated with vehicle (iv) only (twice a week for two weeks). Mice were monitored daily for mortality and clinical signs, body weights were evaluated twice a week. Blood aliquots were collected on days 30 and 31 to determine the percentage of circulating leukemic cells by FACS analysis. BD Cellquest software was used for data collection and analysis. Median survival time (MST) was calculated and the log-rank test was used to evaluate the statistical significance of differences between the control group vs. the quinacrine-treated group.
Results
In the in vitro drug combination analysis, several promising synergies were observed after studying duplicated experiments, for instance when combining quinacrine with geldanamycin, ATRA, cytarabine, hypomethylating agents or sorafenib.In the AML mouse model, evaluation of circulating leukemic cells detected in blood samples (in percent of white blood cells) showed 72 % human tumor cells in the control mice, whereas in mice treated with quinacrine, this was only 2.2%. In agreement with these data, the MST of control mice was 34 days whereas it was 46 days in quinacrine-treated mice (p < 0.0001). At the tested dose, quinacrine did not decrease the body weight of treated mice.
Conclusion
These results strengthen the repositioning potential for quinacrine in AML, suggesting in vivo efficacy as well as promising synergies in combination with different agents, including geldanamycin, conventional chemotherapeutics as well as the hypomethylating agent azacitidine. These results provide further support for evaluation of the anti-leukemic effect of quinacrine in a clinical trial in AML. References1.Eriksson A, Osterroos A, Hassan S, et al. Drug screen in patient cells suggests quinacrine to be repositioned for treatment of acute myeloid leukemia. Blood cancer journal 2015; 5: e307.

Session topic: E-poster
Keyword(s): AML
Type: Eposter Presentation
Background
We have previously reported that quinacrine, formerly extensively used as an antimalarial drug, may have repositioning potential for treatment of acute myeloid leukemia (AML)1.
Aims
The aim of this study was to further evaluate the potential of quinacrine for a clinical trial in AML by investigating its possible synergistic effect with other antileukemic compounds as well as evaluating its efficacy in vivo in a mouse model.
Methods
Cytotoxic activity of quinacrine in combination with one of 9 different drugs (daunorubicin, cytarabine, azacitidine, decitabine, sorafenib, geldanamycin, All-Trans Retinoic Acid (ATRA), vorinostat, and arsenic trioxide) in AML cell lines was evaluated using the fluorometric microculture cytotoxicity assay (FMCA). Assessment of combinatorial effects and possible synergy was performed using conventional Bliss independence analysis based on results from all combinations of 4 different concentrations (including zero concentration) of each drug.Activity of quinacrine in vivo was investigated in a model with AML-PS cells injected intravenously in female SCID mice (at Accelera S.r.l). After two days, groups of ten mice were randomized to treatment with quinacrine or vehicle control. Quinacrine was administered by oral gavage at the dose of 100 mg/kg three times a week for two consecutive weeks; control animals were treated with vehicle (iv) only (twice a week for two weeks). Mice were monitored daily for mortality and clinical signs, body weights were evaluated twice a week. Blood aliquots were collected on days 30 and 31 to determine the percentage of circulating leukemic cells by FACS analysis. BD Cellquest software was used for data collection and analysis. Median survival time (MST) was calculated and the log-rank test was used to evaluate the statistical significance of differences between the control group vs. the quinacrine-treated group.
Results
In the in vitro drug combination analysis, several promising synergies were observed after studying duplicated experiments, for instance when combining quinacrine with geldanamycin, ATRA, cytarabine, hypomethylating agents or sorafenib.In the AML mouse model, evaluation of circulating leukemic cells detected in blood samples (in percent of white blood cells) showed 72 % human tumor cells in the control mice, whereas in mice treated with quinacrine, this was only 2.2%. In agreement with these data, the MST of control mice was 34 days whereas it was 46 days in quinacrine-treated mice (p < 0.0001). At the tested dose, quinacrine did not decrease the body weight of treated mice.
Conclusion
These results strengthen the repositioning potential for quinacrine in AML, suggesting in vivo efficacy as well as promising synergies in combination with different agents, including geldanamycin, conventional chemotherapeutics as well as the hypomethylating agent azacitidine. These results provide further support for evaluation of the anti-leukemic effect of quinacrine in a clinical trial in AML. References1.Eriksson A, Osterroos A, Hassan S, et al. Drug screen in patient cells suggests quinacrine to be repositioned for treatment of acute myeloid leukemia. Blood cancer journal 2015; 5: e307.

Session topic: E-poster
Keyword(s): AML
{{ help_message }}
{{filter}}


