CHARACTERISTICS OF PONATINIB THERAPY FOR PHILADELPHIA-POSITIVE ACUTE LYMPHOBLASTIC LEUKEMIA (PH+ ALL) PATIENTS IN REAL-WORLD CLINICAL PRACTICE COMPARED TO THE PACE TRIAL
(Abstract release date: 05/19/16)
EHA Library. Mauro M. 06/09/16; 132418; E869

Dr. Michael Mauro
Contributions
Contributions
Abstract
Abstract: E869
Type: Eposter Presentation
Background
The pivotal phase 2 PACE trial (NCT01207440) studied the use of ponatinib in adult patients with refractory Ph+ ALL and formed the basis of the approval of ponatinib. Prescribing data tracked by the specialty pharmacy exclusively responsible for distribution of ponatinib in the US are available from Jan 2014 onward.
Aims
To compare the PACE clinical trial data vs real-world pharmacy data, in order to examine similarities and differences between patient characteristics, regimens and duration of therapy evolving over time.
Methods
We compared PACE data, which enrolled patients Sep 2010 – Oct 2011 (all providing informed consent) to real-world data for Ph+ ALL patients starting ponatinib treatment Jan 2014 – Dec 2015. Real-world data source includes referring physicians, pharmacy intake forms and dispensing records. Data on co-prescribing were available for a small subset of patients. Patient characteristics and dosing were compared using non-parametric tests; average daily dose was calculated, including therapy gaps as “zero” dose. Duration of therapy was assessed using Kaplan-Meier techniques and proportional hazard regression.
Results
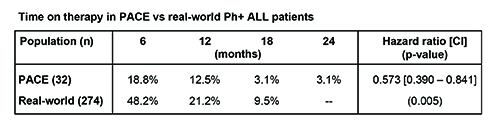
PACE enrolled 32 Ph+ ALL patients; 274 US real-world Ph+ ALL patients started treatment with ponatinib over a 2-year period. Demographic characteristics of PACE vs real-world patients, including age (median 61.5 vs 55.5 years; p=0.589) were similar. Most PACE patients were in their 3rd line of TKI therapy or later (19% 2nd, 44% 3rd and 38% 4th) while most real-world patients appear to be in earlier lines of TKI (29% no prior TKI reported, 32% 2nd line, 24% 3rd, and 15% 4th) (p<0.001). All PACE patients received 45 mg/day of ponatinib as their initial dose; in the real-world, 50% of patients initially received 45 mg/day of ponatinib, 41% received 30 mg/day and 9% 15 mg/day. Average dose was higher in PACE vs real-world (39.1 vs 27.3 mg/day; p<0.001). PACE only permitted monotherapy ponatinib whereas combination therapy appears to be used in a portion of real-world practice. Median time on therapy was 2.7 months in PACE vs 5.5 months in real-world patients (p=0.004), and nearly 50% of real-world patients remained on therapy after 6 months, vs <20% in PACE (Table).
Conclusion
Real-world Ph+ ALL patients appear to be demographically similar to those enrolled in PACE, but there is evidence of ponatinib use earlier (less prior TKI treatment) in real-world patients. Starting dose in real-world reflects 50% <45 mg daily and subsequent lower dose intensity. Median duration of therapy was significantly longer for real-world patients than for PACE, with nearly one-half of real-world patients remaining on therapy at 6 months. Longer median duration of therapy may be related to lower dose intensity, use in earlier therapy lines, and use of combination therapy in real-world clinical practice. Further study of real-world use of ponatinib in Ph+ ALL is needed.
Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, Clinical trial, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
The pivotal phase 2 PACE trial (NCT01207440) studied the use of ponatinib in adult patients with refractory Ph+ ALL and formed the basis of the approval of ponatinib. Prescribing data tracked by the specialty pharmacy exclusively responsible for distribution of ponatinib in the US are available from Jan 2014 onward.
Aims
To compare the PACE clinical trial data vs real-world pharmacy data, in order to examine similarities and differences between patient characteristics, regimens and duration of therapy evolving over time.
Methods
We compared PACE data, which enrolled patients Sep 2010 – Oct 2011 (all providing informed consent) to real-world data for Ph+ ALL patients starting ponatinib treatment Jan 2014 – Dec 2015. Real-world data source includes referring physicians, pharmacy intake forms and dispensing records. Data on co-prescribing were available for a small subset of patients. Patient characteristics and dosing were compared using non-parametric tests; average daily dose was calculated, including therapy gaps as “zero” dose. Duration of therapy was assessed using Kaplan-Meier techniques and proportional hazard regression.
Results
PACE enrolled 32 Ph+ ALL patients; 274 US real-world Ph+ ALL patients started treatment with ponatinib over a 2-year period. Demographic characteristics of PACE vs real-world patients, including age (median 61.5 vs 55.5 years; p=0.589) were similar. Most PACE patients were in their 3rd line of TKI therapy or later (19% 2nd, 44% 3rd and 38% 4th) while most real-world patients appear to be in earlier lines of TKI (29% no prior TKI reported, 32% 2nd line, 24% 3rd, and 15% 4th) (p<0.001). All PACE patients received 45 mg/day of ponatinib as their initial dose; in the real-world, 50% of patients initially received 45 mg/day of ponatinib, 41% received 30 mg/day and 9% 15 mg/day. Average dose was higher in PACE vs real-world (39.1 vs 27.3 mg/day; p<0.001). PACE only permitted monotherapy ponatinib whereas combination therapy appears to be used in a portion of real-world practice. Median time on therapy was 2.7 months in PACE vs 5.5 months in real-world patients (p=0.004), and nearly 50% of real-world patients remained on therapy after 6 months, vs <20% in PACE (Table).
Conclusion
Real-world Ph+ ALL patients appear to be demographically similar to those enrolled in PACE, but there is evidence of ponatinib use earlier (less prior TKI treatment) in real-world patients. Starting dose in real-world reflects 50% <45 mg daily and subsequent lower dose intensity. Median duration of therapy was significantly longer for real-world patients than for PACE, with nearly one-half of real-world patients remaining on therapy at 6 months. Longer median duration of therapy may be related to lower dose intensity, use in earlier therapy lines, and use of combination therapy in real-world clinical practice. Further study of real-world use of ponatinib in Ph+ ALL is needed.

Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, Clinical trial, Tyrosine kinase inhibitor
Abstract: E869
Type: Eposter Presentation
Background
The pivotal phase 2 PACE trial (NCT01207440) studied the use of ponatinib in adult patients with refractory Ph+ ALL and formed the basis of the approval of ponatinib. Prescribing data tracked by the specialty pharmacy exclusively responsible for distribution of ponatinib in the US are available from Jan 2014 onward.
Aims
To compare the PACE clinical trial data vs real-world pharmacy data, in order to examine similarities and differences between patient characteristics, regimens and duration of therapy evolving over time.
Methods
We compared PACE data, which enrolled patients Sep 2010 – Oct 2011 (all providing informed consent) to real-world data for Ph+ ALL patients starting ponatinib treatment Jan 2014 – Dec 2015. Real-world data source includes referring physicians, pharmacy intake forms and dispensing records. Data on co-prescribing were available for a small subset of patients. Patient characteristics and dosing were compared using non-parametric tests; average daily dose was calculated, including therapy gaps as “zero” dose. Duration of therapy was assessed using Kaplan-Meier techniques and proportional hazard regression.
Results
PACE enrolled 32 Ph+ ALL patients; 274 US real-world Ph+ ALL patients started treatment with ponatinib over a 2-year period. Demographic characteristics of PACE vs real-world patients, including age (median 61.5 vs 55.5 years; p=0.589) were similar. Most PACE patients were in their 3rd line of TKI therapy or later (19% 2nd, 44% 3rd and 38% 4th) while most real-world patients appear to be in earlier lines of TKI (29% no prior TKI reported, 32% 2nd line, 24% 3rd, and 15% 4th) (p<0.001). All PACE patients received 45 mg/day of ponatinib as their initial dose; in the real-world, 50% of patients initially received 45 mg/day of ponatinib, 41% received 30 mg/day and 9% 15 mg/day. Average dose was higher in PACE vs real-world (39.1 vs 27.3 mg/day; p<0.001). PACE only permitted monotherapy ponatinib whereas combination therapy appears to be used in a portion of real-world practice. Median time on therapy was 2.7 months in PACE vs 5.5 months in real-world patients (p=0.004), and nearly 50% of real-world patients remained on therapy after 6 months, vs <20% in PACE (Table).
Conclusion
Real-world Ph+ ALL patients appear to be demographically similar to those enrolled in PACE, but there is evidence of ponatinib use earlier (less prior TKI treatment) in real-world patients. Starting dose in real-world reflects 50% <45 mg daily and subsequent lower dose intensity. Median duration of therapy was significantly longer for real-world patients than for PACE, with nearly one-half of real-world patients remaining on therapy at 6 months. Longer median duration of therapy may be related to lower dose intensity, use in earlier therapy lines, and use of combination therapy in real-world clinical practice. Further study of real-world use of ponatinib in Ph+ ALL is needed.

Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, Clinical trial, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
The pivotal phase 2 PACE trial (NCT01207440) studied the use of ponatinib in adult patients with refractory Ph+ ALL and formed the basis of the approval of ponatinib. Prescribing data tracked by the specialty pharmacy exclusively responsible for distribution of ponatinib in the US are available from Jan 2014 onward.
Aims
To compare the PACE clinical trial data vs real-world pharmacy data, in order to examine similarities and differences between patient characteristics, regimens and duration of therapy evolving over time.
Methods
We compared PACE data, which enrolled patients Sep 2010 – Oct 2011 (all providing informed consent) to real-world data for Ph+ ALL patients starting ponatinib treatment Jan 2014 – Dec 2015. Real-world data source includes referring physicians, pharmacy intake forms and dispensing records. Data on co-prescribing were available for a small subset of patients. Patient characteristics and dosing were compared using non-parametric tests; average daily dose was calculated, including therapy gaps as “zero” dose. Duration of therapy was assessed using Kaplan-Meier techniques and proportional hazard regression.
Results
PACE enrolled 32 Ph+ ALL patients; 274 US real-world Ph+ ALL patients started treatment with ponatinib over a 2-year period. Demographic characteristics of PACE vs real-world patients, including age (median 61.5 vs 55.5 years; p=0.589) were similar. Most PACE patients were in their 3rd line of TKI therapy or later (19% 2nd, 44% 3rd and 38% 4th) while most real-world patients appear to be in earlier lines of TKI (29% no prior TKI reported, 32% 2nd line, 24% 3rd, and 15% 4th) (p<0.001). All PACE patients received 45 mg/day of ponatinib as their initial dose; in the real-world, 50% of patients initially received 45 mg/day of ponatinib, 41% received 30 mg/day and 9% 15 mg/day. Average dose was higher in PACE vs real-world (39.1 vs 27.3 mg/day; p<0.001). PACE only permitted monotherapy ponatinib whereas combination therapy appears to be used in a portion of real-world practice. Median time on therapy was 2.7 months in PACE vs 5.5 months in real-world patients (p=0.004), and nearly 50% of real-world patients remained on therapy after 6 months, vs <20% in PACE (Table).
Conclusion
Real-world Ph+ ALL patients appear to be demographically similar to those enrolled in PACE, but there is evidence of ponatinib use earlier (less prior TKI treatment) in real-world patients. Starting dose in real-world reflects 50% <45 mg daily and subsequent lower dose intensity. Median duration of therapy was significantly longer for real-world patients than for PACE, with nearly one-half of real-world patients remaining on therapy at 6 months. Longer median duration of therapy may be related to lower dose intensity, use in earlier therapy lines, and use of combination therapy in real-world clinical practice. Further study of real-world use of ponatinib in Ph+ ALL is needed.

Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, Clinical trial, Tyrosine kinase inhibitor
{{ help_message }}
{{filter}}


