SERIAL MONITORING OF BCR-ABL BY REAL-TIME POLYMERASE CHAIN REACTION IN NEWLY DIAGNOSED PHILADELPHIA CHROMOSOME-POSITIVE ACUTE LYMPHOBLASTIC LEUKEMIA TREATED WITH IMATINIB
(Abstract release date: 05/19/16)
EHA Library. Lim S. 06/09/16; 132405; E856

Dr. Sungnam Lim
Contributions
Contributions
Abstract
Abstract: E856
Type: Eposter Presentation
Background
The positive impact of imatinib on treatment outcome in patients with Philadelphia Chromosome-Positive (Ph+) acute lymphoblastic leukemia (ALL) is well known, and the kinetics of the BCR-ABL transcript correlated with the patient’s clinical course.
Aims
The aim of this study was to evaluate the relationship between imatinib dose intensity and molecular response of BCR-ABL.
Methods
Imatinib (600 mg/day orally) was administered continuously with combination chemotherapy, starting from eighth day of remission induction treatment, then through 5 courses of consolidation or until allogeneic hematopoietic cell transplantation (HCT). Patients who were not transplanted were maintained on imatinib for 2 years. Molecular response monitoring was performed at the central lab with quantitative RT-PCR assays for peripheral blood or bone marrow BCR-ABL RNA in serial; at the time of diagnosis, at hematologic complete remission (HCR), and every 3 months thereafter. The molecular response was defined as complete (MCR) if the BCR-ABL/G6PDH ratio was less than 1x10-5.
Results
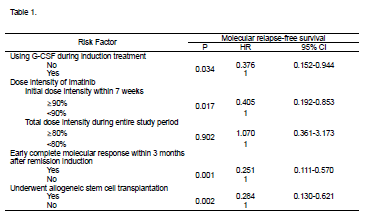
Between October 2005 and February 2009, total 87 patients, aged 16-71 years, with newly diagnosed Ph+ALL were enrolled. With median follow-up of 5 years among survivors (range: 2.6-8.9 years). At the time of diagnosis, the BCR-ABL transcript amount showed no correlation with WBC count and peripheral blast percent. Eighty-two patients (94%) achieved HCR at a median 25 days (range, 14-69 days) and 45 patients (57.7%) of 78 evaluable patients achieved MCR at the same time. Within three months after induction treatment, 59 patients (67.8%) achieved molecular complete remission. Total MCR rate was 88.5% during the entire study period and the median time from treatment to MCR was 54 days (range, 13-384 days). Among these 77 MCR patients, 32 experienced molecular recurrence which was defined as BCR-ABL transcript positive conversion from 1x105. On subsequent follow-up, regardless of allogeneic HCT, 24 additional patients achieved MCR at 3 to 9 months. Of 54 patients who underwent allogeneic HCT while in first CR, with quantitative PCR being performed at a median 2.1 months (range, 0.1-4.0 months) prior to allogeneic HCT, 43 (80%) showed BCR-ABL transcript amount below 1x105. After allogeneic HCT, 49 of 50 evaluated patients (98%) achieved MCR. Median time of MCR duration was 13 months (range, 0.9-60.3 months) and median molecular relapse-free survival was 28 months. Patients with loss of MCR at any time had a higher cumulative incidence of leukemia relapse and a lower relapse-free survival (RFS). Thirty two patients who lost of MCR had significantly inferior RFS (P<0.0001) and OS (P=0.001) then 41 who maintained MCR. MCR achievement within 3 months after remission induction was significant predictor of RFS (P=0.004) and OS (P=0.003). In univariate analyses, the duration of MCR was affected by using G-CSF during remission induction (P=0.0046), initial dose intensity of imatinib within 7 weeks (P=0.001), ≥90% vs. <90%, total imatinib dose intensity during whole study period, and allogeneic HCT (P=0.002). Multivariate analyses after adjusting the Cox model was described in Table 1.
Conclusion
Prospective assessment of the extent of molecular response and imatinib dose intensity during imatinib-based treatment and post-allogenic HCT is likely to be useful in identifying subgroups of Ph+ALL patients at a high risk of relapse and we will be able to apply the risk-adapted or minimal residual disease based therapeutic approaches for Ph+ALL patients.
Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, BCR-ABL, Imatinib
Type: Eposter Presentation
Background
The positive impact of imatinib on treatment outcome in patients with Philadelphia Chromosome-Positive (Ph+) acute lymphoblastic leukemia (ALL) is well known, and the kinetics of the BCR-ABL transcript correlated with the patient’s clinical course.
Aims
The aim of this study was to evaluate the relationship between imatinib dose intensity and molecular response of BCR-ABL.
Methods
Imatinib (600 mg/day orally) was administered continuously with combination chemotherapy, starting from eighth day of remission induction treatment, then through 5 courses of consolidation or until allogeneic hematopoietic cell transplantation (HCT). Patients who were not transplanted were maintained on imatinib for 2 years. Molecular response monitoring was performed at the central lab with quantitative RT-PCR assays for peripheral blood or bone marrow BCR-ABL RNA in serial; at the time of diagnosis, at hematologic complete remission (HCR), and every 3 months thereafter. The molecular response was defined as complete (MCR) if the BCR-ABL/G6PDH ratio was less than 1x10-5.
Results
Between October 2005 and February 2009, total 87 patients, aged 16-71 years, with newly diagnosed Ph+ALL were enrolled. With median follow-up of 5 years among survivors (range: 2.6-8.9 years). At the time of diagnosis, the BCR-ABL transcript amount showed no correlation with WBC count and peripheral blast percent. Eighty-two patients (94%) achieved HCR at a median 25 days (range, 14-69 days) and 45 patients (57.7%) of 78 evaluable patients achieved MCR at the same time. Within three months after induction treatment, 59 patients (67.8%) achieved molecular complete remission. Total MCR rate was 88.5% during the entire study period and the median time from treatment to MCR was 54 days (range, 13-384 days). Among these 77 MCR patients, 32 experienced molecular recurrence which was defined as BCR-ABL transcript positive conversion from 1x105. On subsequent follow-up, regardless of allogeneic HCT, 24 additional patients achieved MCR at 3 to 9 months. Of 54 patients who underwent allogeneic HCT while in first CR, with quantitative PCR being performed at a median 2.1 months (range, 0.1-4.0 months) prior to allogeneic HCT, 43 (80%) showed BCR-ABL transcript amount below 1x105. After allogeneic HCT, 49 of 50 evaluated patients (98%) achieved MCR. Median time of MCR duration was 13 months (range, 0.9-60.3 months) and median molecular relapse-free survival was 28 months. Patients with loss of MCR at any time had a higher cumulative incidence of leukemia relapse and a lower relapse-free survival (RFS). Thirty two patients who lost of MCR had significantly inferior RFS (P<0.0001) and OS (P=0.001) then 41 who maintained MCR. MCR achievement within 3 months after remission induction was significant predictor of RFS (P=0.004) and OS (P=0.003). In univariate analyses, the duration of MCR was affected by using G-CSF during remission induction (P=0.0046), initial dose intensity of imatinib within 7 weeks (P=0.001), ≥90% vs. <90%, total imatinib dose intensity during whole study period, and allogeneic HCT (P=0.002). Multivariate analyses after adjusting the Cox model was described in Table 1.
Conclusion
Prospective assessment of the extent of molecular response and imatinib dose intensity during imatinib-based treatment and post-allogenic HCT is likely to be useful in identifying subgroups of Ph+ALL patients at a high risk of relapse and we will be able to apply the risk-adapted or minimal residual disease based therapeutic approaches for Ph+ALL patients.

Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, BCR-ABL, Imatinib
Abstract: E856
Type: Eposter Presentation
Background
The positive impact of imatinib on treatment outcome in patients with Philadelphia Chromosome-Positive (Ph+) acute lymphoblastic leukemia (ALL) is well known, and the kinetics of the BCR-ABL transcript correlated with the patient’s clinical course.
Aims
The aim of this study was to evaluate the relationship between imatinib dose intensity and molecular response of BCR-ABL.
Methods
Imatinib (600 mg/day orally) was administered continuously with combination chemotherapy, starting from eighth day of remission induction treatment, then through 5 courses of consolidation or until allogeneic hematopoietic cell transplantation (HCT). Patients who were not transplanted were maintained on imatinib for 2 years. Molecular response monitoring was performed at the central lab with quantitative RT-PCR assays for peripheral blood or bone marrow BCR-ABL RNA in serial; at the time of diagnosis, at hematologic complete remission (HCR), and every 3 months thereafter. The molecular response was defined as complete (MCR) if the BCR-ABL/G6PDH ratio was less than 1x10-5.
Results
Between October 2005 and February 2009, total 87 patients, aged 16-71 years, with newly diagnosed Ph+ALL were enrolled. With median follow-up of 5 years among survivors (range: 2.6-8.9 years). At the time of diagnosis, the BCR-ABL transcript amount showed no correlation with WBC count and peripheral blast percent. Eighty-two patients (94%) achieved HCR at a median 25 days (range, 14-69 days) and 45 patients (57.7%) of 78 evaluable patients achieved MCR at the same time. Within three months after induction treatment, 59 patients (67.8%) achieved molecular complete remission. Total MCR rate was 88.5% during the entire study period and the median time from treatment to MCR was 54 days (range, 13-384 days). Among these 77 MCR patients, 32 experienced molecular recurrence which was defined as BCR-ABL transcript positive conversion from 1x105. On subsequent follow-up, regardless of allogeneic HCT, 24 additional patients achieved MCR at 3 to 9 months. Of 54 patients who underwent allogeneic HCT while in first CR, with quantitative PCR being performed at a median 2.1 months (range, 0.1-4.0 months) prior to allogeneic HCT, 43 (80%) showed BCR-ABL transcript amount below 1x105. After allogeneic HCT, 49 of 50 evaluated patients (98%) achieved MCR. Median time of MCR duration was 13 months (range, 0.9-60.3 months) and median molecular relapse-free survival was 28 months. Patients with loss of MCR at any time had a higher cumulative incidence of leukemia relapse and a lower relapse-free survival (RFS). Thirty two patients who lost of MCR had significantly inferior RFS (P<0.0001) and OS (P=0.001) then 41 who maintained MCR. MCR achievement within 3 months after remission induction was significant predictor of RFS (P=0.004) and OS (P=0.003). In univariate analyses, the duration of MCR was affected by using G-CSF during remission induction (P=0.0046), initial dose intensity of imatinib within 7 weeks (P=0.001), ≥90% vs. <90%, total imatinib dose intensity during whole study period, and allogeneic HCT (P=0.002). Multivariate analyses after adjusting the Cox model was described in Table 1.
Conclusion
Prospective assessment of the extent of molecular response and imatinib dose intensity during imatinib-based treatment and post-allogenic HCT is likely to be useful in identifying subgroups of Ph+ALL patients at a high risk of relapse and we will be able to apply the risk-adapted or minimal residual disease based therapeutic approaches for Ph+ALL patients.

Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, BCR-ABL, Imatinib
Type: Eposter Presentation
Background
The positive impact of imatinib on treatment outcome in patients with Philadelphia Chromosome-Positive (Ph+) acute lymphoblastic leukemia (ALL) is well known, and the kinetics of the BCR-ABL transcript correlated with the patient’s clinical course.
Aims
The aim of this study was to evaluate the relationship between imatinib dose intensity and molecular response of BCR-ABL.
Methods
Imatinib (600 mg/day orally) was administered continuously with combination chemotherapy, starting from eighth day of remission induction treatment, then through 5 courses of consolidation or until allogeneic hematopoietic cell transplantation (HCT). Patients who were not transplanted were maintained on imatinib for 2 years. Molecular response monitoring was performed at the central lab with quantitative RT-PCR assays for peripheral blood or bone marrow BCR-ABL RNA in serial; at the time of diagnosis, at hematologic complete remission (HCR), and every 3 months thereafter. The molecular response was defined as complete (MCR) if the BCR-ABL/G6PDH ratio was less than 1x10-5.
Results
Between October 2005 and February 2009, total 87 patients, aged 16-71 years, with newly diagnosed Ph+ALL were enrolled. With median follow-up of 5 years among survivors (range: 2.6-8.9 years). At the time of diagnosis, the BCR-ABL transcript amount showed no correlation with WBC count and peripheral blast percent. Eighty-two patients (94%) achieved HCR at a median 25 days (range, 14-69 days) and 45 patients (57.7%) of 78 evaluable patients achieved MCR at the same time. Within three months after induction treatment, 59 patients (67.8%) achieved molecular complete remission. Total MCR rate was 88.5% during the entire study period and the median time from treatment to MCR was 54 days (range, 13-384 days). Among these 77 MCR patients, 32 experienced molecular recurrence which was defined as BCR-ABL transcript positive conversion from 1x105. On subsequent follow-up, regardless of allogeneic HCT, 24 additional patients achieved MCR at 3 to 9 months. Of 54 patients who underwent allogeneic HCT while in first CR, with quantitative PCR being performed at a median 2.1 months (range, 0.1-4.0 months) prior to allogeneic HCT, 43 (80%) showed BCR-ABL transcript amount below 1x105. After allogeneic HCT, 49 of 50 evaluated patients (98%) achieved MCR. Median time of MCR duration was 13 months (range, 0.9-60.3 months) and median molecular relapse-free survival was 28 months. Patients with loss of MCR at any time had a higher cumulative incidence of leukemia relapse and a lower relapse-free survival (RFS). Thirty two patients who lost of MCR had significantly inferior RFS (P<0.0001) and OS (P=0.001) then 41 who maintained MCR. MCR achievement within 3 months after remission induction was significant predictor of RFS (P=0.004) and OS (P=0.003). In univariate analyses, the duration of MCR was affected by using G-CSF during remission induction (P=0.0046), initial dose intensity of imatinib within 7 weeks (P=0.001), ≥90% vs. <90%, total imatinib dose intensity during whole study period, and allogeneic HCT (P=0.002). Multivariate analyses after adjusting the Cox model was described in Table 1.
Conclusion
Prospective assessment of the extent of molecular response and imatinib dose intensity during imatinib-based treatment and post-allogenic HCT is likely to be useful in identifying subgroups of Ph+ALL patients at a high risk of relapse and we will be able to apply the risk-adapted or minimal residual disease based therapeutic approaches for Ph+ALL patients.

Session topic: E-poster
Keyword(s): Acute lymphoblastic leukemia, BCR-ABL, Imatinib
{{ help_message }}
{{filter}}


