IDELALISIB SENSITIVITY AND MECHANISMS OF DISEASE PROGRESSION IN RELAPSED TCF3-PBX1 ACUTE LYMPHOBLASTIC LEUKEMIA
(Abstract release date: 05/19/16)
EHA Library. Eldfors S. 06/09/16; 132392; E843

Dr. Samuli Eldfors
Contributions
Contributions
Abstract
Abstract: E843
Type: Eposter Presentation
Background
TCF3-PBX1 (E2A-PBX1) is a recurrent gene fusion in B cell precursor lymphoblastic leukemia (BCP-ALL) resulting from translocation t(1;19)(q23;p13). The majority of human TCF3-PBX1 BCP-ALLs are pre-B-cell receptor positive. TCF3-PBX1 BCP-ALL patients typically respond to chemotherapy; however, many relapse and subsequently develop resistant disease with few effective treatment options. Mechanisms driving disease progression and therapy resistance have not been studied previously in TCF3-PBX1 BCP-ALL.
Aims
Here, we aimed to identify novel options for treating TCF3-PBX1 BCP-ALL by profiling leukemic cells from a 25-year-old male who relapsed after chemotherapy and allogeneic bone marrow transplant. In addition, we sought to identify molecular mechanisms underlying disease pathogenesis and progression.
Methods
Bone marrow (BM) aspirates and a skin biopsy were collected from the index patient at diagnosis and relapse. The sensitivity of BM mononuclear cells was assessed against a library of 302 investigational and approved anti-neoplastic drugs. To understand molecular mechanisms underlying the pathogenesis and progression of the disease, we performed in-depth molecular profiling of the patient samples. Exome sequencing was used to identify somatic mutations and copy number aberrations at diagnosis and relapse, while RNA sequencing was performed to identify aberrant gene expression. Drug classes effective at inhibiting the viability of the patient cells were further investigated using TCF3-PBX1+ BCP-ALL and control cell lines that lacked the fusion.
Results
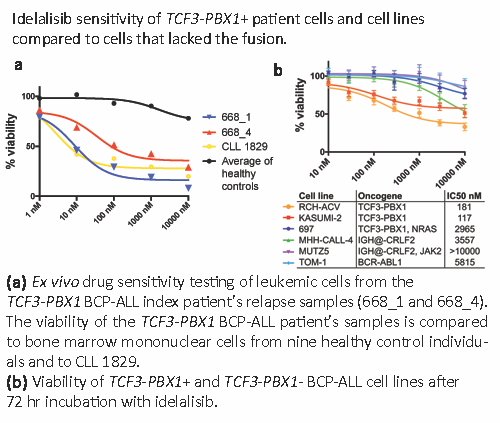
Drug sensitivity testing showed that leukemic blasts from relapse samples of the index patient were sensitive to several classes of targeted drugs. Among the most effective was idelalisib, inhibitor of phosphatidylinositide 3-kinase delta (p110δ) and approved as a second line treatment for CLL and follicular lymphoma. Testing of two relapse samples from the index patient (668_1 and 668_4), positive control CLL and healthy controls showed that the index BCP-ALL and CLL cells were similarly sensitive to idelalisib (Figure 1a). To determine whether idelalisib sensitivity is common to all BCP-ALLs, we tested the sensitivity of TCF3-PBX1 positive (n=3) and negative (n=3) BCP-ALL cell lines. Two TCF3-PBX1+ cells lines were sensitive while all negative cell lines were resistant (Figure 1b). The TCF3-PBX1+ 697 cell line had a mutation to NRAS, likely resulting in RAS pathway activation and altering sensitivity to idelalisib. Idelalisib sensitivity of TCF3-PBX1+ BCP-ALL cells was further supported by evidence showing TCF3-PBX1 directly regulates expression of PIK3CD, the gene encoding p110δ. RNA sequencing of the relapse samples showed high CXCR4 expression, which was not observed in a cohort of diagnostic phase TCF3-PBX1 BCP-ALLs (N=15). CXCR4 mediates interactions with CXL12 expressing BM stromal cells and has been implicated in contact mediated drug resistance. Idelalisib inhibits CXCR4 signaling providing a rationale for using this drug to counter drug resistance. The index patient’s leukemia acquired mutations to both TP53 alleles at relapse. In addition, the patient’s leukemic cells had an MTOR mutation, which was associated with high sensitivity to mTOR inhibitors, which has not been observed before in TCF3-PBX1 BCP-ALL.
Conclusion
Our results suggest idelalisib is a promising treatment option for patients with TCF3-PBX1 BCP-ALL, while other drugs could be useful depending on the genetic context of individual patients.
Session topic: E-poster
Keyword(s): B cell acute lymphoblastic leukemia, CXCR4, PI3K, Relapsed acute lymphoblastic leukemia
Type: Eposter Presentation
Background
TCF3-PBX1 (E2A-PBX1) is a recurrent gene fusion in B cell precursor lymphoblastic leukemia (BCP-ALL) resulting from translocation t(1;19)(q23;p13). The majority of human TCF3-PBX1 BCP-ALLs are pre-B-cell receptor positive. TCF3-PBX1 BCP-ALL patients typically respond to chemotherapy; however, many relapse and subsequently develop resistant disease with few effective treatment options. Mechanisms driving disease progression and therapy resistance have not been studied previously in TCF3-PBX1 BCP-ALL.
Aims
Here, we aimed to identify novel options for treating TCF3-PBX1 BCP-ALL by profiling leukemic cells from a 25-year-old male who relapsed after chemotherapy and allogeneic bone marrow transplant. In addition, we sought to identify molecular mechanisms underlying disease pathogenesis and progression.
Methods
Bone marrow (BM) aspirates and a skin biopsy were collected from the index patient at diagnosis and relapse. The sensitivity of BM mononuclear cells was assessed against a library of 302 investigational and approved anti-neoplastic drugs. To understand molecular mechanisms underlying the pathogenesis and progression of the disease, we performed in-depth molecular profiling of the patient samples. Exome sequencing was used to identify somatic mutations and copy number aberrations at diagnosis and relapse, while RNA sequencing was performed to identify aberrant gene expression. Drug classes effective at inhibiting the viability of the patient cells were further investigated using TCF3-PBX1+ BCP-ALL and control cell lines that lacked the fusion.
Results
Drug sensitivity testing showed that leukemic blasts from relapse samples of the index patient were sensitive to several classes of targeted drugs. Among the most effective was idelalisib, inhibitor of phosphatidylinositide 3-kinase delta (p110δ) and approved as a second line treatment for CLL and follicular lymphoma. Testing of two relapse samples from the index patient (668_1 and 668_4), positive control CLL and healthy controls showed that the index BCP-ALL and CLL cells were similarly sensitive to idelalisib (Figure 1a). To determine whether idelalisib sensitivity is common to all BCP-ALLs, we tested the sensitivity of TCF3-PBX1 positive (n=3) and negative (n=3) BCP-ALL cell lines. Two TCF3-PBX1+ cells lines were sensitive while all negative cell lines were resistant (Figure 1b). The TCF3-PBX1+ 697 cell line had a mutation to NRAS, likely resulting in RAS pathway activation and altering sensitivity to idelalisib. Idelalisib sensitivity of TCF3-PBX1+ BCP-ALL cells was further supported by evidence showing TCF3-PBX1 directly regulates expression of PIK3CD, the gene encoding p110δ. RNA sequencing of the relapse samples showed high CXCR4 expression, which was not observed in a cohort of diagnostic phase TCF3-PBX1 BCP-ALLs (N=15). CXCR4 mediates interactions with CXL12 expressing BM stromal cells and has been implicated in contact mediated drug resistance. Idelalisib inhibits CXCR4 signaling providing a rationale for using this drug to counter drug resistance. The index patient’s leukemia acquired mutations to both TP53 alleles at relapse. In addition, the patient’s leukemic cells had an MTOR mutation, which was associated with high sensitivity to mTOR inhibitors, which has not been observed before in TCF3-PBX1 BCP-ALL.
Conclusion
Our results suggest idelalisib is a promising treatment option for patients with TCF3-PBX1 BCP-ALL, while other drugs could be useful depending on the genetic context of individual patients.

Session topic: E-poster
Keyword(s): B cell acute lymphoblastic leukemia, CXCR4, PI3K, Relapsed acute lymphoblastic leukemia
Abstract: E843
Type: Eposter Presentation
Background
TCF3-PBX1 (E2A-PBX1) is a recurrent gene fusion in B cell precursor lymphoblastic leukemia (BCP-ALL) resulting from translocation t(1;19)(q23;p13). The majority of human TCF3-PBX1 BCP-ALLs are pre-B-cell receptor positive. TCF3-PBX1 BCP-ALL patients typically respond to chemotherapy; however, many relapse and subsequently develop resistant disease with few effective treatment options. Mechanisms driving disease progression and therapy resistance have not been studied previously in TCF3-PBX1 BCP-ALL.
Aims
Here, we aimed to identify novel options for treating TCF3-PBX1 BCP-ALL by profiling leukemic cells from a 25-year-old male who relapsed after chemotherapy and allogeneic bone marrow transplant. In addition, we sought to identify molecular mechanisms underlying disease pathogenesis and progression.
Methods
Bone marrow (BM) aspirates and a skin biopsy were collected from the index patient at diagnosis and relapse. The sensitivity of BM mononuclear cells was assessed against a library of 302 investigational and approved anti-neoplastic drugs. To understand molecular mechanisms underlying the pathogenesis and progression of the disease, we performed in-depth molecular profiling of the patient samples. Exome sequencing was used to identify somatic mutations and copy number aberrations at diagnosis and relapse, while RNA sequencing was performed to identify aberrant gene expression. Drug classes effective at inhibiting the viability of the patient cells were further investigated using TCF3-PBX1+ BCP-ALL and control cell lines that lacked the fusion.
Results
Drug sensitivity testing showed that leukemic blasts from relapse samples of the index patient were sensitive to several classes of targeted drugs. Among the most effective was idelalisib, inhibitor of phosphatidylinositide 3-kinase delta (p110δ) and approved as a second line treatment for CLL and follicular lymphoma. Testing of two relapse samples from the index patient (668_1 and 668_4), positive control CLL and healthy controls showed that the index BCP-ALL and CLL cells were similarly sensitive to idelalisib (Figure 1a). To determine whether idelalisib sensitivity is common to all BCP-ALLs, we tested the sensitivity of TCF3-PBX1 positive (n=3) and negative (n=3) BCP-ALL cell lines. Two TCF3-PBX1+ cells lines were sensitive while all negative cell lines were resistant (Figure 1b). The TCF3-PBX1+ 697 cell line had a mutation to NRAS, likely resulting in RAS pathway activation and altering sensitivity to idelalisib. Idelalisib sensitivity of TCF3-PBX1+ BCP-ALL cells was further supported by evidence showing TCF3-PBX1 directly regulates expression of PIK3CD, the gene encoding p110δ. RNA sequencing of the relapse samples showed high CXCR4 expression, which was not observed in a cohort of diagnostic phase TCF3-PBX1 BCP-ALLs (N=15). CXCR4 mediates interactions with CXL12 expressing BM stromal cells and has been implicated in contact mediated drug resistance. Idelalisib inhibits CXCR4 signaling providing a rationale for using this drug to counter drug resistance. The index patient’s leukemia acquired mutations to both TP53 alleles at relapse. In addition, the patient’s leukemic cells had an MTOR mutation, which was associated with high sensitivity to mTOR inhibitors, which has not been observed before in TCF3-PBX1 BCP-ALL.
Conclusion
Our results suggest idelalisib is a promising treatment option for patients with TCF3-PBX1 BCP-ALL, while other drugs could be useful depending on the genetic context of individual patients.

Session topic: E-poster
Keyword(s): B cell acute lymphoblastic leukemia, CXCR4, PI3K, Relapsed acute lymphoblastic leukemia
Type: Eposter Presentation
Background
TCF3-PBX1 (E2A-PBX1) is a recurrent gene fusion in B cell precursor lymphoblastic leukemia (BCP-ALL) resulting from translocation t(1;19)(q23;p13). The majority of human TCF3-PBX1 BCP-ALLs are pre-B-cell receptor positive. TCF3-PBX1 BCP-ALL patients typically respond to chemotherapy; however, many relapse and subsequently develop resistant disease with few effective treatment options. Mechanisms driving disease progression and therapy resistance have not been studied previously in TCF3-PBX1 BCP-ALL.
Aims
Here, we aimed to identify novel options for treating TCF3-PBX1 BCP-ALL by profiling leukemic cells from a 25-year-old male who relapsed after chemotherapy and allogeneic bone marrow transplant. In addition, we sought to identify molecular mechanisms underlying disease pathogenesis and progression.
Methods
Bone marrow (BM) aspirates and a skin biopsy were collected from the index patient at diagnosis and relapse. The sensitivity of BM mononuclear cells was assessed against a library of 302 investigational and approved anti-neoplastic drugs. To understand molecular mechanisms underlying the pathogenesis and progression of the disease, we performed in-depth molecular profiling of the patient samples. Exome sequencing was used to identify somatic mutations and copy number aberrations at diagnosis and relapse, while RNA sequencing was performed to identify aberrant gene expression. Drug classes effective at inhibiting the viability of the patient cells were further investigated using TCF3-PBX1+ BCP-ALL and control cell lines that lacked the fusion.
Results
Drug sensitivity testing showed that leukemic blasts from relapse samples of the index patient were sensitive to several classes of targeted drugs. Among the most effective was idelalisib, inhibitor of phosphatidylinositide 3-kinase delta (p110δ) and approved as a second line treatment for CLL and follicular lymphoma. Testing of two relapse samples from the index patient (668_1 and 668_4), positive control CLL and healthy controls showed that the index BCP-ALL and CLL cells were similarly sensitive to idelalisib (Figure 1a). To determine whether idelalisib sensitivity is common to all BCP-ALLs, we tested the sensitivity of TCF3-PBX1 positive (n=3) and negative (n=3) BCP-ALL cell lines. Two TCF3-PBX1+ cells lines were sensitive while all negative cell lines were resistant (Figure 1b). The TCF3-PBX1+ 697 cell line had a mutation to NRAS, likely resulting in RAS pathway activation and altering sensitivity to idelalisib. Idelalisib sensitivity of TCF3-PBX1+ BCP-ALL cells was further supported by evidence showing TCF3-PBX1 directly regulates expression of PIK3CD, the gene encoding p110δ. RNA sequencing of the relapse samples showed high CXCR4 expression, which was not observed in a cohort of diagnostic phase TCF3-PBX1 BCP-ALLs (N=15). CXCR4 mediates interactions with CXL12 expressing BM stromal cells and has been implicated in contact mediated drug resistance. Idelalisib inhibits CXCR4 signaling providing a rationale for using this drug to counter drug resistance. The index patient’s leukemia acquired mutations to both TP53 alleles at relapse. In addition, the patient’s leukemic cells had an MTOR mutation, which was associated with high sensitivity to mTOR inhibitors, which has not been observed before in TCF3-PBX1 BCP-ALL.
Conclusion
Our results suggest idelalisib is a promising treatment option for patients with TCF3-PBX1 BCP-ALL, while other drugs could be useful depending on the genetic context of individual patients.

Session topic: E-poster
Keyword(s): B cell acute lymphoblastic leukemia, CXCR4, PI3K, Relapsed acute lymphoblastic leukemia
{{ help_message }}
{{filter}}


