Hematology, Oncology, and Clinical Immunology

Contributions
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:15 to 13.06.2015 16:30
Location: Room C1
Background
Prognostication in myelodysplastic syndromes (MDS) has recently been improved by the revised International Prognostic Scoring System (IPSS-R). However this score, as the original IPSS, was developed analyzing primary, untreated patients (pts) only. Data on its usefulness in pts with therapy-related MDS (tMDS) is limited.
Aims
We analyzed 615 pts from Spanish, German, Swiss, and Austrian centers diagnosed 1975-2015.
Methods
Complete data to calculate the IPSS/-R was available in 446 pts. Prognostic impact of features was analyzed by uni- and multivariable models and estimated by a measure of concordance for censored data (Dxy).
Results
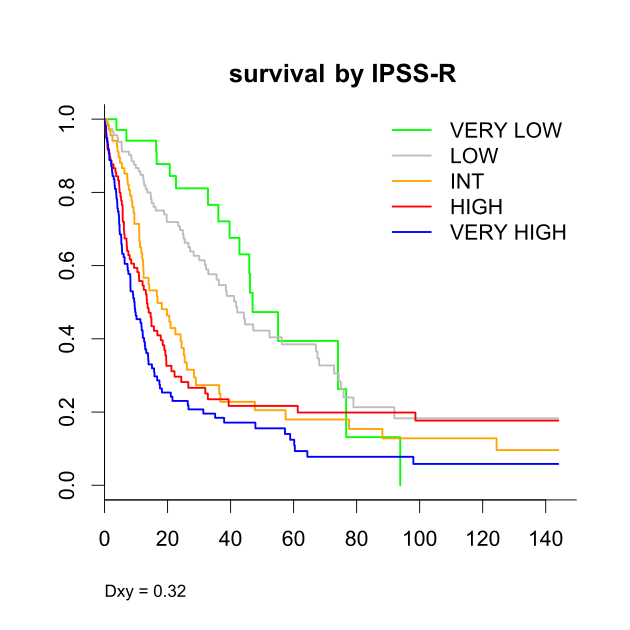
: Median age was 67 years. According to WHO classification 2% of pts had 5q-syndrome, 6% RA, 3% RARS, 27% RCMD, 9% RCMD-RS, 16% RAEB-1, 18% RAEB-2, 6% CMML-1, 2% CMML-2, 3% MDS-U, and 8% AML (RAEB-T). Cytogenetics were 47% good, 14% intermediate, and 39% poor according to IPSS, and 2% very good, 44% good, 17% intermediate, 16% poor, and 21% very poor according to IPSS-R. Regarding prognostic risk groups 19% exhibited IPSS-low, 33% int-1, 30% int-2, and 18% high, while the IPSS-R was very low in 8%, low in 26%, intermediate in 16%, high in 22%, and very high in 27%.
Regarding the primary disease most frequent diagnoses were NHL 19%, breast cancer 16%, myeloma 10%, Hodgkin’s disease, and AML 6% each. 75% of pts received chemotherapy and 47% received radiotherapy. Most pts received combination regimen containing alkylating agents in 59%, topoisomerase inhibitors in 35%, antitubulin agents in 28%, and antimetabolites in 38%. Latency periods varied broadly (≤3 yrs 22%, >3-≤6 yrs 26%, >6-≤12 yrs 31%, >12 yrs 21%).
Median follow-up from MDS diagnosis was 56 months, median survival 17 months. After MDS diagnosis 30% of pts received disease altering treatment, including stem cell transplantation in 17%.
Features with influence on survival and time to AML in univariable analysis included age, FAB, WHO, IPSS, IPSS-R, cytogenetic risk, platelets, marrow and peripheral blasts, ferritin, fibrosis, year of primary diagnosis. Predominantly influence on survival was seen for year of MDS diagnosis, hemoglobin, LDH, and use of alkylating agents. A latency period >12 years showed higher risk of AML. Neutrophil count, use of chemo or radiotherapy as well as other chemotherapeutic agents had no influence on both outcomes.
Our results indicate that both the IPSS (Dxy 0.26 for survival, 0.35 for AML), and IPSS-R (Dxy 0.32 for both) perform moderately in tMDS, but not as well as in primary MDS. Adjusting prognostic models to tMDS seems therefore required. Score versions including peripheral blasts perform somewhat better. Separate score versions for survival and time to AML would give differing weights to most features. Hemoglobin and cytogenetics would get more weight for survival, while marrow blasts would be more important regarding AML. Another issue is the possible integration of data on primary disease/therapy.
Summary
In contrast to early publications on tMDS, where aberrant cytogenetics were described in >90% of pts and prognosis was seen uniformly poor, surprisingly we find good risk karyotypes in a relatively large number. Although some cases might be unrelated to previous therapy and poor risk cytogenetics are still overrepresented, this indicates, different types of tMDS exist. Our analysis shows that indeed many variables exhibit a prognostic influence in tMDS. About one third of our pts were treated for MDS. However, censoring/leaving them out would not show a representative cohort. Further analyses are performed to propose an optimized scoring system for tMDS.
Keyword(s): Myelodysplasia, Prognostic factor
Session topic: MDS Clinical
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:15 to 13.06.2015 16:30
Location: Room C1
Background
Prognostication in myelodysplastic syndromes (MDS) has recently been improved by the revised International Prognostic Scoring System (IPSS-R). However this score, as the original IPSS, was developed analyzing primary, untreated patients (pts) only. Data on its usefulness in pts with therapy-related MDS (tMDS) is limited.
Aims
We analyzed 615 pts from Spanish, German, Swiss, and Austrian centers diagnosed 1975-2015.
Methods
Complete data to calculate the IPSS/-R was available in 446 pts. Prognostic impact of features was analyzed by uni- and multivariable models and estimated by a measure of concordance for censored data (Dxy).
Results
: Median age was 67 years. According to WHO classification 2% of pts had 5q-syndrome, 6% RA, 3% RARS, 27% RCMD, 9% RCMD-RS, 16% RAEB-1, 18% RAEB-2, 6% CMML-1, 2% CMML-2, 3% MDS-U, and 8% AML (RAEB-T). Cytogenetics were 47% good, 14% intermediate, and 39% poor according to IPSS, and 2% very good, 44% good, 17% intermediate, 16% poor, and 21% very poor according to IPSS-R. Regarding prognostic risk groups 19% exhibited IPSS-low, 33% int-1, 30% int-2, and 18% high, while the IPSS-R was very low in 8%, low in 26%, intermediate in 16%, high in 22%, and very high in 27%.
Regarding the primary disease most frequent diagnoses were NHL 19%, breast cancer 16%, myeloma 10%, Hodgkin’s disease, and AML 6% each. 75% of pts received chemotherapy and 47% received radiotherapy. Most pts received combination regimen containing alkylating agents in 59%, topoisomerase inhibitors in 35%, antitubulin agents in 28%, and antimetabolites in 38%. Latency periods varied broadly (≤3 yrs 22%, >3-≤6 yrs 26%, >6-≤12 yrs 31%, >12 yrs 21%).
Median follow-up from MDS diagnosis was 56 months, median survival 17 months. After MDS diagnosis 30% of pts received disease altering treatment, including stem cell transplantation in 17%.
Features with influence on survival and time to AML in univariable analysis included age, FAB, WHO, IPSS, IPSS-R, cytogenetic risk, platelets, marrow and peripheral blasts, ferritin, fibrosis, year of primary diagnosis. Predominantly influence on survival was seen for year of MDS diagnosis, hemoglobin, LDH, and use of alkylating agents. A latency period >12 years showed higher risk of AML. Neutrophil count, use of chemo or radiotherapy as well as other chemotherapeutic agents had no influence on both outcomes.
Our results indicate that both the IPSS (Dxy 0.26 for survival, 0.35 for AML), and IPSS-R (Dxy 0.32 for both) perform moderately in tMDS, but not as well as in primary MDS. Adjusting prognostic models to tMDS seems therefore required. Score versions including peripheral blasts perform somewhat better. Separate score versions for survival and time to AML would give differing weights to most features. Hemoglobin and cytogenetics would get more weight for survival, while marrow blasts would be more important regarding AML. Another issue is the possible integration of data on primary disease/therapy.
Summary
In contrast to early publications on tMDS, where aberrant cytogenetics were described in >90% of pts and prognosis was seen uniformly poor, surprisingly we find good risk karyotypes in a relatively large number. Although some cases might be unrelated to previous therapy and poor risk cytogenetics are still overrepresented, this indicates, different types of tMDS exist. Our analysis shows that indeed many variables exhibit a prognostic influence in tMDS. About one third of our pts were treated for MDS. However, censoring/leaving them out would not show a representative cohort. Further analyses are performed to propose an optimized scoring system for tMDS.
Keyword(s): Myelodysplasia, Prognostic factor

Session topic: MDS Clinical


