Hematología

Contributions
Type: Oral Presentation + travel grant
Presentation during EHA20: From 14.06.2015 09:00 to 14.06.2015 09:15
Location: Room Lehar 1 + 2
Background
High-risk Follicular Lymphoma (FL) patients intensified with high dose therapy and autologous stem cell transplantation HDT/ASCT may achieve prolonged remissions. The best timing for the procedure remains controversial. Addition of rituximab to conventional chemotherapy has brought a significant development, with several phase III randomized trials proving a benefit in favor of a rituximab-containing chemotherapy up front. The vast majority of studies of HDT/ASCT in FL have been performed in the pre-rituximab era, and its value now remains to be elucidated.
Aims
To analyze the impact of previos Rituximab exposure in patients intensified with HDT/ASCT in a retrospective study from the Spanish Geltamo Registry.
Methods
All FL patients undergoing HDT/ASCT from 1989 to 2007 and reported to the Spanish GELTAMO registry (n=655, mean age 47 years, male sex 49,6%) were eligible for this study. 203 patients (31%) received HDT/ASCT after achievement of 1st CR, 43% of them requiring more than one therapy line to achieve CR; 26% in 2ndCR, 5% in 3rdCR, 21,5% in 1stPR, 12,5% in chemo sensitive recurrence, and 5% with active disease. Of the 312 cases who were evaluable for FLIPI, 125 (38%) were in the high-risk group. Of the 332 who were evaluable for FLIPI II, 125 (40%) were in in the high-risk group. Of the 640 assessable for Rituximab exposure previously to ASCT, 184 (30%) had been treated with the anti-CD20 monoclonal antibody (R+ Group). Overall survival (OS) and progression free survival (PFS) probabilities were calculated using Kaplan-Meier estimates. The log rank test was used for univariate comparisons.
Results
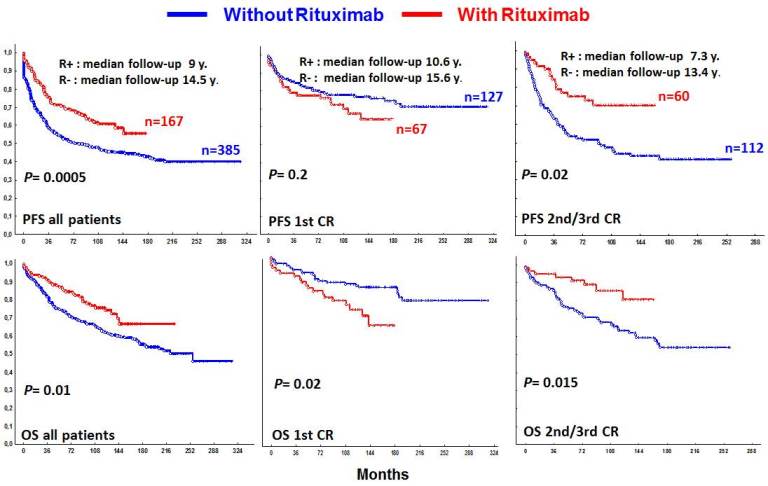
Median follow-up from HDT/ASCT was 14, 5 years for the Rituximab-naïve (R- Group) patients and 9 years for the R+ Group. In the R+ Group, patients were older (P=0.01) and there were more with ECOG >1 (P=0.05), with high LDH (P=0.02), with poor risk FLIPI II (P=0.05) and had a shorter length of follow-up (P<0.0001). In patients transplanted in 1st CR, there were more patients who received more than one line of therapy to reach CR in the R+ Group (P=0.02). Globally, R+ Group showed a better outcome than R-Group (median PFS not reached vs 74 months (P=0.0006); median OS not reached vs 221 months, respectively; (P=0, 02)). For patients transplanted in 2nd/3rd CR the benefit of Rituximab was remarkable: median PFS not reached vs 92 months (P=0.002); OS at 5 years 91%, OS at 10 years 86%, in the R+ Group vs OS at 5 years 76%, OS at 10 years 63% for the R- Group, respectively (P=0.01). Surprisingly this benefit did not occur in patients transplanted in 1st CR, where R- patients showed even a better outcome (no statistically differences on PFS and OS at 5 years 91% vs 83%, OS at 10 years 84% vs 72% for the R- Group and R+ Group, respectively;(P=0.02)).
Summary
Follicular lymphoma patients undergoing HDT/ASCT in 2nd / 3rd CR have a formidable outcome; treatment with rituximab seems to have a synergistic effect with HDT/ASCT in this population. R+ Group transplanted in 1st CR, showed an excellent outcome, however it was no better than the obtained in R- Group. Worse initial prognostic factors in the R+ Group, with more patients needing more than one line of therapy to reach the CR, could explain these data.
In the Rituximab era, HDT/ ASCT should be considered in the therapeutic approach at first relapse for FL patients. Very high risk patients could, as well, benefit from a transplant in 1st CR. As well-balanced randomized studies are not easily feasible in this setting, we think that updating the results of the available studies could elucidate this question.
Keyword(s): Autologous hematopoietic stem cell transplantation, Follicular lymphoma, Rituximab
Session topic: Stem cell transplantation: Clinical 3
Type: Oral Presentation + travel grant
Presentation during EHA20: From 14.06.2015 09:00 to 14.06.2015 09:15
Location: Room Lehar 1 + 2
Background
High-risk Follicular Lymphoma (FL) patients intensified with high dose therapy and autologous stem cell transplantation HDT/ASCT may achieve prolonged remissions. The best timing for the procedure remains controversial. Addition of rituximab to conventional chemotherapy has brought a significant development, with several phase III randomized trials proving a benefit in favor of a rituximab-containing chemotherapy up front. The vast majority of studies of HDT/ASCT in FL have been performed in the pre-rituximab era, and its value now remains to be elucidated.
Aims
To analyze the impact of previos Rituximab exposure in patients intensified with HDT/ASCT in a retrospective study from the Spanish Geltamo Registry.
Methods
All FL patients undergoing HDT/ASCT from 1989 to 2007 and reported to the Spanish GELTAMO registry (n=655, mean age 47 years, male sex 49,6%) were eligible for this study. 203 patients (31%) received HDT/ASCT after achievement of 1st CR, 43% of them requiring more than one therapy line to achieve CR; 26% in 2ndCR, 5% in 3rdCR, 21,5% in 1stPR, 12,5% in chemo sensitive recurrence, and 5% with active disease. Of the 312 cases who were evaluable for FLIPI, 125 (38%) were in the high-risk group. Of the 332 who were evaluable for FLIPI II, 125 (40%) were in in the high-risk group. Of the 640 assessable for Rituximab exposure previously to ASCT, 184 (30%) had been treated with the anti-CD20 monoclonal antibody (R+ Group). Overall survival (OS) and progression free survival (PFS) probabilities were calculated using Kaplan-Meier estimates. The log rank test was used for univariate comparisons.
Results
Median follow-up from HDT/ASCT was 14, 5 years for the Rituximab-naïve (R- Group) patients and 9 years for the R+ Group. In the R+ Group, patients were older (P=0.01) and there were more with ECOG >1 (P=0.05), with high LDH (P=0.02), with poor risk FLIPI II (P=0.05) and had a shorter length of follow-up (P<0.0001). In patients transplanted in 1st CR, there were more patients who received more than one line of therapy to reach CR in the R+ Group (P=0.02). Globally, R+ Group showed a better outcome than R-Group (median PFS not reached vs 74 months (P=0.0006); median OS not reached vs 221 months, respectively; (P=0, 02)). For patients transplanted in 2nd/3rd CR the benefit of Rituximab was remarkable: median PFS not reached vs 92 months (P=0.002); OS at 5 years 91%, OS at 10 years 86%, in the R+ Group vs OS at 5 years 76%, OS at 10 years 63% for the R- Group, respectively (P=0.01). Surprisingly this benefit did not occur in patients transplanted in 1st CR, where R- patients showed even a better outcome (no statistically differences on PFS and OS at 5 years 91% vs 83%, OS at 10 years 84% vs 72% for the R- Group and R+ Group, respectively;(P=0.02)).
Summary
Follicular lymphoma patients undergoing HDT/ASCT in 2nd / 3rd CR have a formidable outcome; treatment with rituximab seems to have a synergistic effect with HDT/ASCT in this population. R+ Group transplanted in 1st CR, showed an excellent outcome, however it was no better than the obtained in R- Group. Worse initial prognostic factors in the R+ Group, with more patients needing more than one line of therapy to reach the CR, could explain these data.
In the Rituximab era, HDT/ ASCT should be considered in the therapeutic approach at first relapse for FL patients. Very high risk patients could, as well, benefit from a transplant in 1st CR. As well-balanced randomized studies are not easily feasible in this setting, we think that updating the results of the available studies could elucidate this question.
Keyword(s): Autologous hematopoietic stem cell transplantation, Follicular lymphoma, Rituximab

Session topic: Stem cell transplantation: Clinical 3


