UPDATED RESULTS OF A MULTICENTER PHASE I/IB STUDY OF CTLA4 BLOCKADE WITH IPILIMUMAB FOR RELAPSED HEMATOLOGIC MALIGNANCIES AFTER ALLOGENEIC HEMATOPOIETIC CELL TRANSPLANTATION
(Abstract release date: 05/21/15)
EHA Library. Davids M. 06/13/15; 103200; S445
Disclosure(s): Dana-Farber Cancer InstituteMedical Oncology

Dr. Matthew Davids
Contributions
Contributions
Abstract
Abstract: S445
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:30 to 13.06.2015 12:45
Location: Room C2
Background
Patients with hematologic malignancies (HM) who relapse after alloHCT often have a dampened graft versus tumor (GVT) effect and limited treatment options. Immune checkpoint modulation with CTLA4 blockade could be a novel pharmacologic strategy to augment GVT to restore the anti-tumor activity of the graft.
Aims
This is an ongoing multicenter phase I/Ib study of the CTLA4 blocking antibody ipilimumab to treat patients (pts) with HM of any histology who relapse after alloHCT. The primary aims are to determine the MTD and evaluate safety. Secondary aims include assessments of efficacy and changes in immune cell phenotype.
Methods
After informed consent was obtained, ipilimumab was given off-label at 3 mg/kg or 10 mg/kg IV every 3 weeks for 4 cycles of induction, followed by maintenance dosing every 12 weeks up to 1 year. Disease-specific response criteria were assessed at the mid-point (7 weeks), end of induction (13 weeks), and throughout maintenance. Immunophenotyping was performed by 8-color flow cytometry and analyzed by FACSDiva.
Results
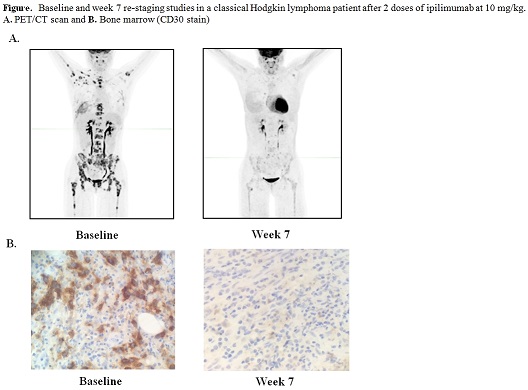
Twenty-seven pts have received treatment to date. In phase I, 6 pts were treated at 3 mg/kg and 7 pts were treated at 10 mg/kg. An MTD was not reached, and 14 pts subsequently enrolled in the phase Ib expansion cohort at 10 mg/kg. The median number of prior therapies excluding transplant was 3 (range 2-11), and 19/27 (70.4%) of pts had received prior therapy for post transplant relapse. Histologies included AML (n=11), cHL (n=7), NHL (n=4), and MDS (n=2), and 1 pt each had MM, MPN, and ALL. The median age at enrollment was 58 yrs. (range 22-75). Immune-related adverse events (irAEs) were observed in 4 pts, including pneumonitis (n=1 gr2, n=2 gr4), diarrhea (n=2 gr 1), ITP (n=1 gr2), and colitis (n=1 gr 3), and were reversible with steroids, with 3 pts able to resume ipilimumab. Four DLTs leading to discontinuation have been observed, including cGVHD (n=2, both liver, gr 3), aGVHD (n=1, gut, gr 2), and TRM (n=1) due to presumed sepsis in the context of severe irAEs. Seventeen patients discontinued due to progressive disease, and 6 patients remain on active treatment. In an interim efficacy analysis, while none of the 5 evaluable pts treated at 3 mg/kg responded, 8 of the 20 (40.0%) evaluable pts treated at 10 mg/kg had anti-tumor activity. Six pts achieved formal response by disease-specific criteria, including a cHL patient with a PR with dramatic reduction in nodal and extranodal disease and a marrow CR at 7 weeks (see figure), a MM pt with a PR with near resolution of a lung plasmacytoma, and four pts with AML who achieved CR, including two with leukemia cutis and one with myeloid sarcoma. The median follow-up time among survivors is 5.5 mo., and 6 mo. OS is currently 66%. Immunophenotyping studies revealed that while the absolute numbers of both Treg and Tconv cells increased slightly after ipilimumab, the ratio of Treg/Tconv decreased (range 24% to 41% decrease).
Summary
While some toxicity including irAEs and GVHD was seen with ipilimumab 10 mg/kg in pts with relapsed HM after alloHCT, substantial anti-tumor activity was also observed in pts with both lymphoid and myeloid malignanices, including highly chemorefractory diseases such as leukemia cutis and myeloid sarcoma. The Treg/Tconv cell ratio decreased with treatment, consistent with enhancement of GVT. Immune checkpoint modulation with CTLA4 blockade may be a promising new therapeutic approach for post alloHCT relapse, and is worthy of exploration in future studies.
Keyword(s): Allogeneic hematopoietic stem cell transplant, CTLA-4, Graft-versus-host disease (GVHD), Graft-versus-tumor effect
Session topic: Stem cell transplantation: Clinical 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:30 to 13.06.2015 12:45
Location: Room C2
Background
Patients with hematologic malignancies (HM) who relapse after alloHCT often have a dampened graft versus tumor (GVT) effect and limited treatment options. Immune checkpoint modulation with CTLA4 blockade could be a novel pharmacologic strategy to augment GVT to restore the anti-tumor activity of the graft.
Aims
This is an ongoing multicenter phase I/Ib study of the CTLA4 blocking antibody ipilimumab to treat patients (pts) with HM of any histology who relapse after alloHCT. The primary aims are to determine the MTD and evaluate safety. Secondary aims include assessments of efficacy and changes in immune cell phenotype.
Methods
After informed consent was obtained, ipilimumab was given off-label at 3 mg/kg or 10 mg/kg IV every 3 weeks for 4 cycles of induction, followed by maintenance dosing every 12 weeks up to 1 year. Disease-specific response criteria were assessed at the mid-point (7 weeks), end of induction (13 weeks), and throughout maintenance. Immunophenotyping was performed by 8-color flow cytometry and analyzed by FACSDiva.
Results
Twenty-seven pts have received treatment to date. In phase I, 6 pts were treated at 3 mg/kg and 7 pts were treated at 10 mg/kg. An MTD was not reached, and 14 pts subsequently enrolled in the phase Ib expansion cohort at 10 mg/kg. The median number of prior therapies excluding transplant was 3 (range 2-11), and 19/27 (70.4%) of pts had received prior therapy for post transplant relapse. Histologies included AML (n=11), cHL (n=7), NHL (n=4), and MDS (n=2), and 1 pt each had MM, MPN, and ALL. The median age at enrollment was 58 yrs. (range 22-75). Immune-related adverse events (irAEs) were observed in 4 pts, including pneumonitis (n=1 gr2, n=2 gr4), diarrhea (n=2 gr 1), ITP (n=1 gr2), and colitis (n=1 gr 3), and were reversible with steroids, with 3 pts able to resume ipilimumab. Four DLTs leading to discontinuation have been observed, including cGVHD (n=2, both liver, gr 3), aGVHD (n=1, gut, gr 2), and TRM (n=1) due to presumed sepsis in the context of severe irAEs. Seventeen patients discontinued due to progressive disease, and 6 patients remain on active treatment. In an interim efficacy analysis, while none of the 5 evaluable pts treated at 3 mg/kg responded, 8 of the 20 (40.0%) evaluable pts treated at 10 mg/kg had anti-tumor activity. Six pts achieved formal response by disease-specific criteria, including a cHL patient with a PR with dramatic reduction in nodal and extranodal disease and a marrow CR at 7 weeks (see figure), a MM pt with a PR with near resolution of a lung plasmacytoma, and four pts with AML who achieved CR, including two with leukemia cutis and one with myeloid sarcoma. The median follow-up time among survivors is 5.5 mo., and 6 mo. OS is currently 66%. Immunophenotyping studies revealed that while the absolute numbers of both Treg and Tconv cells increased slightly after ipilimumab, the ratio of Treg/Tconv decreased (range 24% to 41% decrease).
Summary
While some toxicity including irAEs and GVHD was seen with ipilimumab 10 mg/kg in pts with relapsed HM after alloHCT, substantial anti-tumor activity was also observed in pts with both lymphoid and myeloid malignanices, including highly chemorefractory diseases such as leukemia cutis and myeloid sarcoma. The Treg/Tconv cell ratio decreased with treatment, consistent with enhancement of GVT. Immune checkpoint modulation with CTLA4 blockade may be a promising new therapeutic approach for post alloHCT relapse, and is worthy of exploration in future studies.
Keyword(s): Allogeneic hematopoietic stem cell transplant, CTLA-4, Graft-versus-host disease (GVHD), Graft-versus-tumor effect

Session topic: Stem cell transplantation: Clinical 2
Abstract: S445
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:30 to 13.06.2015 12:45
Location: Room C2
Background
Patients with hematologic malignancies (HM) who relapse after alloHCT often have a dampened graft versus tumor (GVT) effect and limited treatment options. Immune checkpoint modulation with CTLA4 blockade could be a novel pharmacologic strategy to augment GVT to restore the anti-tumor activity of the graft.
Aims
This is an ongoing multicenter phase I/Ib study of the CTLA4 blocking antibody ipilimumab to treat patients (pts) with HM of any histology who relapse after alloHCT. The primary aims are to determine the MTD and evaluate safety. Secondary aims include assessments of efficacy and changes in immune cell phenotype.
Methods
After informed consent was obtained, ipilimumab was given off-label at 3 mg/kg or 10 mg/kg IV every 3 weeks for 4 cycles of induction, followed by maintenance dosing every 12 weeks up to 1 year. Disease-specific response criteria were assessed at the mid-point (7 weeks), end of induction (13 weeks), and throughout maintenance. Immunophenotyping was performed by 8-color flow cytometry and analyzed by FACSDiva.
Results
Twenty-seven pts have received treatment to date. In phase I, 6 pts were treated at 3 mg/kg and 7 pts were treated at 10 mg/kg. An MTD was not reached, and 14 pts subsequently enrolled in the phase Ib expansion cohort at 10 mg/kg. The median number of prior therapies excluding transplant was 3 (range 2-11), and 19/27 (70.4%) of pts had received prior therapy for post transplant relapse. Histologies included AML (n=11), cHL (n=7), NHL (n=4), and MDS (n=2), and 1 pt each had MM, MPN, and ALL. The median age at enrollment was 58 yrs. (range 22-75). Immune-related adverse events (irAEs) were observed in 4 pts, including pneumonitis (n=1 gr2, n=2 gr4), diarrhea (n=2 gr 1), ITP (n=1 gr2), and colitis (n=1 gr 3), and were reversible with steroids, with 3 pts able to resume ipilimumab. Four DLTs leading to discontinuation have been observed, including cGVHD (n=2, both liver, gr 3), aGVHD (n=1, gut, gr 2), and TRM (n=1) due to presumed sepsis in the context of severe irAEs. Seventeen patients discontinued due to progressive disease, and 6 patients remain on active treatment. In an interim efficacy analysis, while none of the 5 evaluable pts treated at 3 mg/kg responded, 8 of the 20 (40.0%) evaluable pts treated at 10 mg/kg had anti-tumor activity. Six pts achieved formal response by disease-specific criteria, including a cHL patient with a PR with dramatic reduction in nodal and extranodal disease and a marrow CR at 7 weeks (see figure), a MM pt with a PR with near resolution of a lung plasmacytoma, and four pts with AML who achieved CR, including two with leukemia cutis and one with myeloid sarcoma. The median follow-up time among survivors is 5.5 mo., and 6 mo. OS is currently 66%. Immunophenotyping studies revealed that while the absolute numbers of both Treg and Tconv cells increased slightly after ipilimumab, the ratio of Treg/Tconv decreased (range 24% to 41% decrease).
Summary
While some toxicity including irAEs and GVHD was seen with ipilimumab 10 mg/kg in pts with relapsed HM after alloHCT, substantial anti-tumor activity was also observed in pts with both lymphoid and myeloid malignanices, including highly chemorefractory diseases such as leukemia cutis and myeloid sarcoma. The Treg/Tconv cell ratio decreased with treatment, consistent with enhancement of GVT. Immune checkpoint modulation with CTLA4 blockade may be a promising new therapeutic approach for post alloHCT relapse, and is worthy of exploration in future studies.
Keyword(s): Allogeneic hematopoietic stem cell transplant, CTLA-4, Graft-versus-host disease (GVHD), Graft-versus-tumor effect

Session topic: Stem cell transplantation: Clinical 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:30 to 13.06.2015 12:45
Location: Room C2
Background
Patients with hematologic malignancies (HM) who relapse after alloHCT often have a dampened graft versus tumor (GVT) effect and limited treatment options. Immune checkpoint modulation with CTLA4 blockade could be a novel pharmacologic strategy to augment GVT to restore the anti-tumor activity of the graft.
Aims
This is an ongoing multicenter phase I/Ib study of the CTLA4 blocking antibody ipilimumab to treat patients (pts) with HM of any histology who relapse after alloHCT. The primary aims are to determine the MTD and evaluate safety. Secondary aims include assessments of efficacy and changes in immune cell phenotype.
Methods
After informed consent was obtained, ipilimumab was given off-label at 3 mg/kg or 10 mg/kg IV every 3 weeks for 4 cycles of induction, followed by maintenance dosing every 12 weeks up to 1 year. Disease-specific response criteria were assessed at the mid-point (7 weeks), end of induction (13 weeks), and throughout maintenance. Immunophenotyping was performed by 8-color flow cytometry and analyzed by FACSDiva.
Results
Twenty-seven pts have received treatment to date. In phase I, 6 pts were treated at 3 mg/kg and 7 pts were treated at 10 mg/kg. An MTD was not reached, and 14 pts subsequently enrolled in the phase Ib expansion cohort at 10 mg/kg. The median number of prior therapies excluding transplant was 3 (range 2-11), and 19/27 (70.4%) of pts had received prior therapy for post transplant relapse. Histologies included AML (n=11), cHL (n=7), NHL (n=4), and MDS (n=2), and 1 pt each had MM, MPN, and ALL. The median age at enrollment was 58 yrs. (range 22-75). Immune-related adverse events (irAEs) were observed in 4 pts, including pneumonitis (n=1 gr2, n=2 gr4), diarrhea (n=2 gr 1), ITP (n=1 gr2), and colitis (n=1 gr 3), and were reversible with steroids, with 3 pts able to resume ipilimumab. Four DLTs leading to discontinuation have been observed, including cGVHD (n=2, both liver, gr 3), aGVHD (n=1, gut, gr 2), and TRM (n=1) due to presumed sepsis in the context of severe irAEs. Seventeen patients discontinued due to progressive disease, and 6 patients remain on active treatment. In an interim efficacy analysis, while none of the 5 evaluable pts treated at 3 mg/kg responded, 8 of the 20 (40.0%) evaluable pts treated at 10 mg/kg had anti-tumor activity. Six pts achieved formal response by disease-specific criteria, including a cHL patient with a PR with dramatic reduction in nodal and extranodal disease and a marrow CR at 7 weeks (see figure), a MM pt with a PR with near resolution of a lung plasmacytoma, and four pts with AML who achieved CR, including two with leukemia cutis and one with myeloid sarcoma. The median follow-up time among survivors is 5.5 mo., and 6 mo. OS is currently 66%. Immunophenotyping studies revealed that while the absolute numbers of both Treg and Tconv cells increased slightly after ipilimumab, the ratio of Treg/Tconv decreased (range 24% to 41% decrease).
Summary
While some toxicity including irAEs and GVHD was seen with ipilimumab 10 mg/kg in pts with relapsed HM after alloHCT, substantial anti-tumor activity was also observed in pts with both lymphoid and myeloid malignanices, including highly chemorefractory diseases such as leukemia cutis and myeloid sarcoma. The Treg/Tconv cell ratio decreased with treatment, consistent with enhancement of GVT. Immune checkpoint modulation with CTLA4 blockade may be a promising new therapeutic approach for post alloHCT relapse, and is worthy of exploration in future studies.
Keyword(s): Allogeneic hematopoietic stem cell transplant, CTLA-4, Graft-versus-host disease (GVHD), Graft-versus-tumor effect

Session topic: Stem cell transplantation: Clinical 2
{{ help_message }}
{{filter}}


