HMDS

Contributions
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 09:00 to 14.06.2015 09:15
Location: Room A7
Background
Minimal residual disease (MRD) in CLL is an independent predictor of progression-free (PFS) and overall survival (OS) with a high, intermediate and low risk of disease progression seen in patients with >1%, 0.01-1%, or <0.01% MRD respectively. Evaluating novel treatments currently requires assessment of PFS/OS in randomised controlled trials but the prolonged duration of remission means that this approach is slow. Assessing MRD as a trial endpoint could greatly speed the process; using peripheral blood (PB) would be preferable but antibody therapies preferentially deplete circulating disease to a variable extent and bone marrow (BM) is the more sensitive site for MRD detection.
Aims
To compare PFS/OS according to MRD level in PB and BM for different treatment and patient groups and determine the optimum site for MRD assessment.
Methods
MRD was quantified using multi-parameter flow cytometry according to ERIC consensus protocols with a limit of quantification of 10-4 / 0.01% or better.
Results
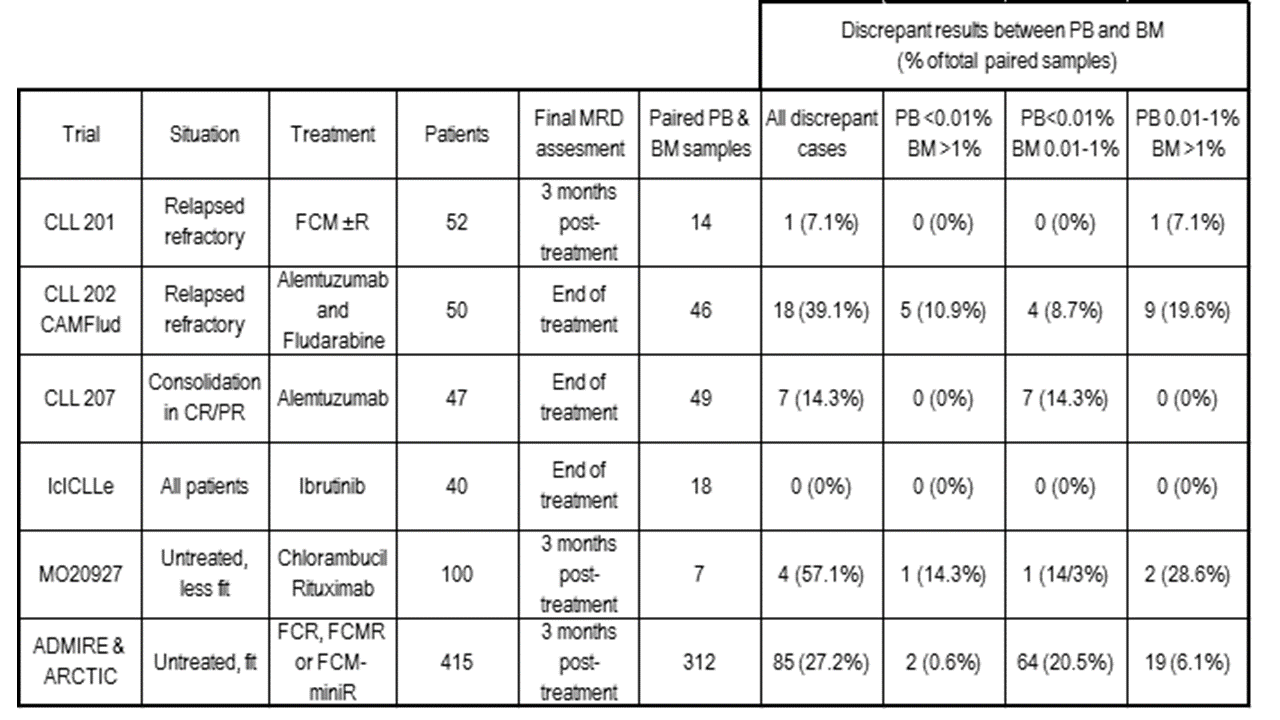
The MRD level in 609 paired PB & BM samples taken during or within 6 months of treatment (446 in clinical trials, see table) was concordant in 443 (72.7%) at MRD thresholds <0.01% / 0.01-1% / >1%. In 391 cases with <0.01% PB MRD, 85/391 (21.7%) had 0.01-1% CLL and 14/292 (3.6%) had >1% CLL in the paired BM sample. In 118 cases with 0.01-1% CLL in the PB, the paired BM sample showed >1% CLL in 63/118 (53.4%). The PB MRD level would have assigned a higher risk category than BM in only 4 cases, all of which had detectable BM disease.
Discrepancies between PB & BM MRD levels were not consistent across treatment and patient groups. Refractory patients treated with alemtuzumab-based therapies typically showed a high proportion of discrepant results with >1 log differences while patients in CR/PR receiving alemtuzumab consolidation had few discrepancies; during Ibrutinib monotherapy (IcICLLE trial) there have been no discordant cases to date.
To determine whether discrepant results impact on outcome, PFS/OS was evaluated according to PB & BM MRD in different trials. In the ADMIRE/ARCTIC trials, PB & BM MRD levels were discordant in 66/312 evaluable cases, all with ≥0.01% BM but <0.01% PB MRD. After a median follow-up of 3 years, MRD level was confirmed as a strong predictor of PFS and OS, independent of categorical response, with the presence of ≥0.01% MRD conferring a significantly poorer outcome (PFS: HR 4.6, 95%CI 2.7 to 7.7, p=<0.001). For patients in CR, and with ≥0.01% MRD (n=70), 3 year PFS was 68.7% compared with 86.6% for patients with <0.01% MRD (n=176). There was no observable difference in the PFS or OS for patients with <0.01% PB but ≥0.01% BM MRD compared to those with <0.01% PB & BM MRD. However, for those patients with MRD detected in the BM only, there was a median of 0.08% CLL and this would be predicted to yield observable differences in PFS only after >3 years follow-up. The results contrast with the outcome for 47 evaluable patients in the CLL202 CAMFlud trial where patients with <0.01% MRD in PB but ≥0.01% disease in BM had a poor outcome that was similar to those with ≥0.01% in both PB & BM, and both groups had significantly poorer PFS/OS than patients with <0.01% MRD in both PB & BM (with median 55 months follow-up for patients with ≥0.01% in PB & BM / <0.01% PB but ≥0.01% BM / <0.01% MRD in both PB & BM patients respectively, median PFS was 1.6 / 6.5 / 44.7 months and OS was 7.5 / 14 / not reached, P<0.001).
Summary
Patients achieving <0.01% CLL have a better outcome than those with detectable MRD but the power of peripheral blood MRD eradication to predict outcome varies widely according to the type of therapy received and is poor with more potent antibodies. The most robust tissue and time-point for MRD assessment to predict long-term outcomes in CLL is the bone marrow at 3 months post-treatment. Our work justifies the use of MRD eradication from the marrow post-treatment as a surrogate end-point in clinical trials.
Keyword(s): Bone Marrow, Chronic lymphocytic leukemia, Minimal residual disease (MRD), Peripheral blood
Session topic: CLL: Refining outcomes
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 09:00 to 14.06.2015 09:15
Location: Room A7
Background
Minimal residual disease (MRD) in CLL is an independent predictor of progression-free (PFS) and overall survival (OS) with a high, intermediate and low risk of disease progression seen in patients with >1%, 0.01-1%, or <0.01% MRD respectively. Evaluating novel treatments currently requires assessment of PFS/OS in randomised controlled trials but the prolonged duration of remission means that this approach is slow. Assessing MRD as a trial endpoint could greatly speed the process; using peripheral blood (PB) would be preferable but antibody therapies preferentially deplete circulating disease to a variable extent and bone marrow (BM) is the more sensitive site for MRD detection.
Aims
To compare PFS/OS according to MRD level in PB and BM for different treatment and patient groups and determine the optimum site for MRD assessment.
Methods
MRD was quantified using multi-parameter flow cytometry according to ERIC consensus protocols with a limit of quantification of 10-4 / 0.01% or better.
Results
The MRD level in 609 paired PB & BM samples taken during or within 6 months of treatment (446 in clinical trials, see table) was concordant in 443 (72.7%) at MRD thresholds <0.01% / 0.01-1% / >1%. In 391 cases with <0.01% PB MRD, 85/391 (21.7%) had 0.01-1% CLL and 14/292 (3.6%) had >1% CLL in the paired BM sample. In 118 cases with 0.01-1% CLL in the PB, the paired BM sample showed >1% CLL in 63/118 (53.4%). The PB MRD level would have assigned a higher risk category than BM in only 4 cases, all of which had detectable BM disease.
Discrepancies between PB & BM MRD levels were not consistent across treatment and patient groups. Refractory patients treated with alemtuzumab-based therapies typically showed a high proportion of discrepant results with >1 log differences while patients in CR/PR receiving alemtuzumab consolidation had few discrepancies; during Ibrutinib monotherapy (IcICLLE trial) there have been no discordant cases to date.
To determine whether discrepant results impact on outcome, PFS/OS was evaluated according to PB & BM MRD in different trials. In the ADMIRE/ARCTIC trials, PB & BM MRD levels were discordant in 66/312 evaluable cases, all with ≥0.01% BM but <0.01% PB MRD. After a median follow-up of 3 years, MRD level was confirmed as a strong predictor of PFS and OS, independent of categorical response, with the presence of ≥0.01% MRD conferring a significantly poorer outcome (PFS: HR 4.6, 95%CI 2.7 to 7.7, p=<0.001). For patients in CR, and with ≥0.01% MRD (n=70), 3 year PFS was 68.7% compared with 86.6% for patients with <0.01% MRD (n=176). There was no observable difference in the PFS or OS for patients with <0.01% PB but ≥0.01% BM MRD compared to those with <0.01% PB & BM MRD. However, for those patients with MRD detected in the BM only, there was a median of 0.08% CLL and this would be predicted to yield observable differences in PFS only after >3 years follow-up. The results contrast with the outcome for 47 evaluable patients in the CLL202 CAMFlud trial where patients with <0.01% MRD in PB but ≥0.01% disease in BM had a poor outcome that was similar to those with ≥0.01% in both PB & BM, and both groups had significantly poorer PFS/OS than patients with <0.01% MRD in both PB & BM (with median 55 months follow-up for patients with ≥0.01% in PB & BM / <0.01% PB but ≥0.01% BM / <0.01% MRD in both PB & BM patients respectively, median PFS was 1.6 / 6.5 / 44.7 months and OS was 7.5 / 14 / not reached, P<0.001).
Summary
Patients achieving <0.01% CLL have a better outcome than those with detectable MRD but the power of peripheral blood MRD eradication to predict outcome varies widely according to the type of therapy received and is poor with more potent antibodies. The most robust tissue and time-point for MRD assessment to predict long-term outcomes in CLL is the bone marrow at 3 months post-treatment. Our work justifies the use of MRD eradication from the marrow post-treatment as a surrogate end-point in clinical trials.
Keyword(s): Bone Marrow, Chronic lymphocytic leukemia, Minimal residual disease (MRD), Peripheral blood

Session topic: CLL: Refining outcomes


